Question from June's Chemical Processing
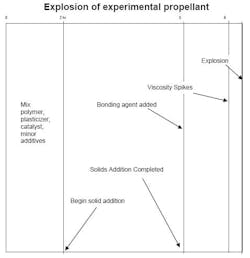
A modified-banbury mixer is used to blend a new solid rocket propellant. It is a DOT class 1.1, double-base, nitroglycerine propellant. During the first step, where only polymer and plasticizer are present, everything went well. HMX and ammonium perchlorate are used in the formulation. Following industry practice, the coarse oxides are added first, then the finer oxides and aluminum. The bonding agent and cross-linker are added normally. The mixer is maintained under a slight vacuum and chilled water cools the side of the vessel. The bottom and top are not chilled. The interior surface of the vessel and mixer are polished to a pharmaceutical finish. Later, as solids are added, approaching 90%, the mixer explodes. Fifteen minutes before the explosion, the viscosity unexpectedly spiked. There was a sharp rise in viscosity after that (Figure 1). Fortunately, nobody was hurt. What caused the incident and what can we do to improve the operation?
Consider a number of possibilities
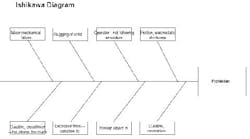
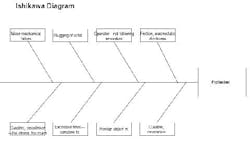
I hope I can offer some insights to your problem. I used to work in the propellant industry, but with J.H. Day mixers, not Banburys. First of all, I’m glad no one was hurt in the incident. Protecting people should be your number one concern. Look at this experience as a learning opportunity to make sure this never happens again. An Ishikawa diagram should help explain my thoughts on the cause of the accident (Figure 2).
Figure 2. This chart highlights various factors that could have led to solid propellant explosion. (Click to enlarge.)
What initiates explosive mixtures? Heat, friction and electrostatic discharge are a likely cause. The mix temperatures may be a clue here. I would review the records to confirm that they were within their normal ranges. Also, the maintenance history of the temperature elements and data acquisition systems should be reviewed to make sure temperature readings were accurate. The grounding procedures of the solid feeding vessels (e.g., sifter) should be confirmed. Make sure the solids feeding vessels were grounded to the mixer, and they were both attached to a building ground, so there would be no build up of electrostatic potential. Interviews with operators should be conducted to make sure they were doing this important step correctly. Feeding fine HMX, AP and aluminum powder can result in large voltages if not grounded correctly.
Next, I would look at the mechanical equipment. How was the mix viscosity monitored? Was it from power demand on the motor? Normally, the reactions of the urethane and the crosslinker cause an exponential increase in the batch viscosity (with time). Handling of the propellant isn’t difficult; casting is usually done well before the material sets up. A viscosity spike is unusual. I assume that quality control is good, i.e., that the isocyanate and crosslinker were checked before adding them to the batch. This leaves only mechanical problems. What could cause the spike? I see two possibilities: a foreign object in the mix or slugging of the solid feed.
Since the blade-to-blade and blade-to-bowl clearances are usually very small in these high-shear mixers, a foreign object could have wedged itself between the blades and accounted for a viscosity spike (if measured by motor power demand). Of course, this could have resulted in high friction which could have initiated the explosion. The investigator should look for paths of foreign material entry to the mixer. For instance, are all solids screened prior to addition? Are all tools accounted for? Do operators empty their pockets prior to entering the mix building?
Solids slugging could result in a viscosity spike. The solids may have rushed into the mixing vessel and caused a temporary increase in power demand. Then, as the solids wetted, power demand would have dropped. Does the mix system have a history of solids slugging? Slugging could result in friction or electrostatic discharge.
Again, getting back to the curative (the isocyanate), there may be a few unexplored problems. Was the proper amount and strength of curative used? Too much curative has caused high mix viscosity and temperature and (if memory serves me correctly) has resulted in mixer fires and explosions. Review the batch records for procedures. Inspect the ingredient lots for curative strength, composition and general condition of the containers and storage area.
Is there any evidence from the debris which could be used to locate the exact spot in the mixer where the explosion initiated? For instance, are their any burn marks near a bearing, on a blade, etc.? Collection and reassembly of the remaining mixer components could be helpful.
Edward Giugliano, team leader
AES Warrior Run, LLC, Cumberland, Md.
An impurity caused a chain reaction
I believe that a polymerization chain reaction occurred. Possibly the heat of mixing or poor heat transfer in the jacket may have set it off. Typically, a chain reaction is blamed on free radicals. A polymerization reaction doesn’t have to involve formation of free radicals to occur. It can be caused by an ionic mechanism, either cationic or anionic. Since aluminum metal is an ingredient in your batch that seems the likely culprit. If some oxidizing potential exists, or existed, whereby the aluminum could have been converted to a cationic agent this could cause the chain reaction. Humidity, combined with the presence of sulfur compounds, like SO3, can oxidize aluminum. There’s an organic reaction that was identified just about 15 years ago called the “manich” which involves ammonia/amines, formaldehyde and carbonyls. If your organics are di-carbonyl functionalized I can see a lot of possibilities for cationic polymerization to occur if there’s any water in the mixture to allow ionic, acidic, species to exist. I think there must have been an impurity in the batch ingredients, possibly from the ammonium perchlorate, aluminum, or HMX, or something else, that may have caused the aluminum to initiate a chain reaction.
Charles Fivelson, engineer
Carbondale, Ill.
Was the curing agent contaminated?
Contaminants can trigger the isocyanate to react with itself to form an isocyanurate, releasing a lot of heat. Typical triggers are potassium and sodium, but iron can coordinate the reaction as well. The metal must be in ionic form, which usually occurs at a particular temperature for a catalyst designed for the reaction (potassium octoate for instance). If the reaction starts in any part of the mixture, it would propagate due to the amount of heat released. This would occur whether there are fillers (the aluminum and oxidizer, i.e., the HMX and ammonium perchlorate) or not. If this is the cause of the explosion, you need to reduce the heat generated in the batch. A polyol can be pre-reacted to reduce this risk. For instance, if the isocyanate is at 24% NCO, react it with about 40% by weight of a high molecular weight polyol like Terathane 2000 (or PEG 2000 to lessen signature) to bring the NCO down to 12%. The exotherm potential will be cut in half and the entropy of the pre-polymer will help retard the isocyanurate reaction.
Another possible cause of the problem might be the metal in the mixer itself. Just before getting my doctorate at Clemson, I interviewed at Redstone Arsenal where a lot of propellant work is done. They mixed their batches in Tupperware to maintain smaller quantities, reduce friction and reduce spark potential. A plastic lined metal container would probably be better for heat transfer though. Another way to reduce this potential would be foaming the mixture with a gas like helium. This would reduce the temperature and greatly reduce shear since a much-lower-density material is being stirred. The problem would be getting the gas out of the polymer, especially as it starts to cure at the end of the batch. Perhaps a vacuum extruder could be used before casting the propellant.
Dr. Jim Yavorsky, consultant
Chemical Consultants Network, Mickleton, N.J.
Reduce the mixer speed or change the formulation
The viscosity rise caused more energy to be expended to mix the material and also reduced heat transfer to the cooled surfaces. A rise in temperature occurred. This rise caused the reaction rates (polymerization and others) to increase until the explosion happened. The mixer speed should be reduced or the propellant formulation should be changed. Lower the viscosity by reducing the solids (aluminum, HMX, or ammonium perchlorate) or increasing the plasticizer to reduce the viscosity. Changing the formulation may require significant development work. Reducing the solids loading will affect combustion efficiency and Isp or “specific impulse.” Isp is the ratio of thrust produced to the weight of the flowing gases. Raising the plasticizer/polymer ratio could be detrimental to long-term storage of the propellant. Both of these potential problems must be considered.
Richard H. Smith, P.E., team leader
Texas Commission on Environmental Quality, Austin, Texas
Change the batch procedure
Being unfamiliar with urethanes I can only speculate. If the viscosity increased, I can imagine that, similar to polymers such as epoxies and hardeners, once they mix, they react and generate heat, expand, etc. It appears that while mixing is occurring, like regular epoxy, heat built up very rapidly, material expanded, etc. as the viscosity increased and formed a chain reaction, due to the high reactivity of the mixture. As material was added and further mixing occurred, it is like adding fuel to a fire — feeding the reaction. If the viscosity rapidly increased, obviously the reaction was advancing at a fast pace as well. Although a slight vacuum was used, when the material was added, it may have acted like a pressure cooker, resulting in an explosion.
One suggestion is to slow the mixing rate, while the cooling is going on and add the later components at a slower rate as well to control the heat, expansion, ignition, etc. This would require some additional steps to the batch procedure but avoid a heat build-up.
Another suggestion, if the cause was an ignition, would be to blanket the mixing with an inert gas, e.g., nitrogen or argon. Of course changing from a vacuum system to a pressure system will require regulators, relief valves, etc. and a review of the vessel pressure stamp. Another advantage of using an inert is how it will solve the problem of trapped air. As the fine particles are added, any air that might be entrapped in these particles (interstitially, or from the process flow during its entry to the mix) won’t matter, since the inert gas is helping to prevent ignition. One problem with a blanket is preventing trapped air, and inert gas, from creating voids in the propellant. Moisture in the air also could cause bubbles to form. A vacuum process is needed. This will require a closed, thin-film extrusion with a vacuum prior to casting the propellant.
Rich Ashley, associate dir. QC/tech affairs
Barr Labs, Pomona, N.Y.
Review the process
Examine the overall system and equipment. Start by reviewing the instrumentation: the RTD, the current loads on the agitator. The mixer exploded because the heat transfer was poor: low conductivities from high viscosities. Go back to the drawing board.
Tom Murphy, CEO
Puritrol, Inc., Centerville, Mass.
October's Puzzler
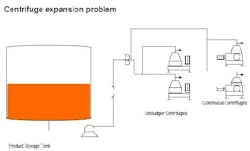
The centrifuge separation at a bio-products plant is a bottleneck. Currently, two desludger centrifuges and two continuous (nozzle) centrifuges are available. The solution must be flexible since several products may be centrifuged. The product slurry concentration varies from 8% to as high as 15% by weight in an aqueous solution. Viscosity varies from 1-20 cP, for some products. Newtonian flow is not guaranteed. The desludgers offer better separation but at half the rate of the continuous units — at the moment one continuous unit can manage plant flow. There is some concern about nozzle selection for the continuous centrifuges; the feeling is that increasing the nozzle size will reduce the centrifuge efficiency but extend the run time between cleanings. 7-mm nozzles are being used but going to 11-mm may only increase the throughput by 10% for the current product. The maximum discharge pressure for the unit is about 50 psig. The maximum loss to byproducts is about 10% of the batch. Note that the typical level, produced from a batch, is about 50% in the product tank. Without buying more units, at about $330K for a continuous unit, how would you eliminate this bottleneck (Figure 3)?
Figure 3. Improve the capacity and quality of centrifuge separation.
Send us your comments, suggestions or solutions for this question by September 8, 2006. We’ll include as many of them as possible in the October 2006 issue and all on CP.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to ProcessPuzzler, Chemical Processing, 555 W. Pierce Rd., Suite 301, Itasca, IL 60143. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you’d like to pose to our readers, send it along and we’ll be pleased to consider it for publication.