Ensuring smooth operation of a crystallization process is often considered an art rather than a science. When problems occur — such as the final product fails to meet the target impurity content or the productivity is much lower than expected — few engineers would go back to the fundamental science, such as phase equilibrium and crystallization kinetics, to gain a better understanding of what causes the problem. Indeed, know-how and experience are invaluable in solving such problems, because crystallization is a complex process where many interrelated phenomena are in play. Nonetheless, a systematic analysis can lead to a significant reduction in time and effort for finding a suitable solution compared to trial and error.
Crystallization involves two key steps: formation of solid particles from liquid solution (normally referred to as nucleation), and growth due to the deposition of additional substance on existing particles. The thermodynamic driving force behind both steps is the difference in chemical potential between solution (liquid phase) and crystal (solid phase). In practice, that difference can be represented by supersaturation, which is defined as the difference between the actual concentration of the crystallizing substance in the solution and its saturation concentration. Many textbooks and articles on crystallization begin with this notion and proceed to discuss various topics such as crystallization kinetics and particle size distribution. While an understanding of such issues is critical in analyzing a crystallization process, do not underestimate the importance of solid/liquid equilibrium (SLE).
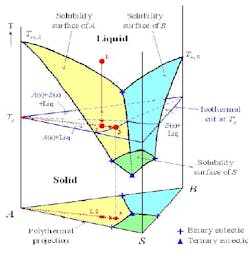
Clearly, supersaturation can only be quantified if the saturation concentration, often referred to as solubility, is known. Because most industrial systems are multi-component in nature, solubility data of pure components are not enough, as solubility depends on the concentration of all other components present in the mixture. Furthermore, it is important to know which components are supersaturated. This issue is frequently overlooked, partly because crystallization is often considered as a purification technique. However, the presence of seemingly insignificant amounts of impurities in the solution can lead to co-crystallization of both the product and the impurity simply because the impurity is much less soluble than the product. For these reasons, understanding the SLE behavior of the entire system is crucial.
The phase diagram
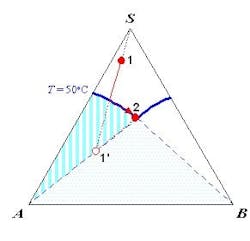
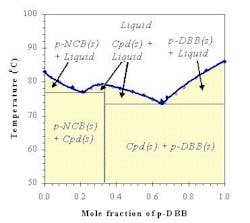
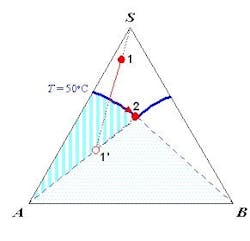
The SLE behavior of a system can be conveniently represented in a phase diagram that shows the regions of composition, temperature and pressure under which the system would exist as a single phase or a mixture of multiple phases. Because SLE behavior is normally insensitive to pressure, it is common practice to focus only on temperature and composition. Figure 1a shows the SLE phase diagram for a two-component system of o-nitrochlorobenzene (o-NCB) and p-nitrochlorobenzene (p-NCB), which exhibits so-called simple eutectic behavior. A mixture containing 56% o-NCB at 50°C (point 1) exists as a liquid, but when cooled down to 20°C (point 2) it would phase split to give crystals of p-NCB and a liquid solution with a composition indicated by point 3, because the overall composition falls within the two-phase region. On the other hand, another mixture containing 80% o-NCB would yield crystals of o-NCB when cooled down to the same temperature, as this composition would be inside the “o-NCB(s) + Liquid” region. Both mixtures would completely turn into solid if they were cooled down further to reach the “p-NCB(s) + o-NCB(s)” region.
Build on a solid foundation
Understanding the root of the problem is essential in troubleshooting a crystallization process, because the success in finding the most appropriate solution depends on the ability to identify the underlying phenomenon that causes the symptoms. When product fails to meet purity requirements, crystallization, inclusion and occlusion of impurities are the three mechanisms to be considered. By studying the SLE behavior and testing the impurity profile, it should be possible to identify the responsible mechanism and take a suitable corrective action without much trial and error.
References
1. Matsuoka, M., “Developments in melt crystallization” in “Advances in industrial crystallization,” J. Garside, R.J. Davey and A.G. Jones, eds., Butterworth-Heinemann, Oxford, U.K. (1991).
2. Wakeman, R.J. and E.S. Tarleton, “Filtration: equipment selection, modelling and process simulation,” Elsevier, Oxford, U.K. (1999).
3. Walas, S.M., “Phase equilibria in chemical engineering,” Butterworths, Boston (1985).
4. Tare, J.P. and M.R. Chivate, “Separation of close boiling isomers by adductive and extractive crystallization,” A.I.Ch.E. Symp. Ser. No. 153, 72, p. 95 (1976).
Further Reading
Wibowo, C. and K.M. Ng, “Unified approach for synthesizing crystallization-based separation processes,” A.I.Ch.E. J., 46, p.1,400 (2000).
Wibowo, C. and K.M. Ng, “Workflow for process synthesis and development: crystallization and solids processing,” Ind. Eng. Chem. Res., 41, p. 3,839 (2002).
Christianto Wibowo is a senior engineer at ClearWaterBay Technology, Inc., Walnut, Calif. E-mail him at [email protected].