Rethink Nitrogen Supply for Chemical Blanketing
Many processors rely on blanketing or padding to protect chemicals stored in tanks against contamination, degradation or chemical change as well as to prevent fire or explosions. Blanketing involves introducing a low-pressure (typically less than a few psig) flow of an inert gas above the liquid level of the chemical to fill the vapor space at the top of the tank. Nitrogen is the most commonly used gas because it's widely available and relatively inexpensive but other gases such as carbon dioxide or argon sometimes are employed. However, carbon dioxide is more reactive than nitrogen and argon is about ten times more expensive.
Typically, tanks with fixed roofs and unsealed tanks are blanketed while those with floating roofs aren't. The 95–99.9%-pure nitrogen blanket or pad creates a slight positive pressure in a closed tank, helping prevent the ingression of ambient air that could cause oxidative degradation or combustion.
A recent accident brings home the importance of tank blanketing. A North American paint manufacturer had used blanketing to protect select solvents but never instituted the technique as a standard practice. A tank not protected by a nitrogen blanket caught fire, resulting in significant damage, downtime and loss of revenue. Fortunately, no one was hurt. Needless to say, the facility soon made tank blanketing a standard practice. Not only does this help protect plant personnel and products, but it also eliminates the need for federal agencies such as the U.S. Chemical Safety and Hazard Review Board to investigate a facility.
Figure 1. Unit features membranes containing bundles of hollow fibers and requires no electricity.
TANK BLANKETING OPTIONSA storage tank can be made inert several ways. One is by reducing the oxygen content in the vapor space to a value lower than the minimum concentration that supports combustion, i.e., the limiting oxygen concentration (LOC). Another is by keeping the fuel concentration in the headspace to a value lower than the minimum concentration that supports combustion, i.e., the lower explosive limit (LEL) or lower flammability limit. The third and final option is to increase the fuel concentration in the headspace to a value greater than the maximum concentration that supports combustion, i.e., the upper explosive limit (UEL) or upper flammability limit. A material's flammability envelope is bounded by its LEL, UEL and LOC. The values for specific chemicals can be found in publications such as material safety data sheets, the National Fire Protection Association's "NFPA 69: Standard on Explosion Prevention Systems" and chemistry handbooks. How nitrogen is controlled usually depends upon the type of tank used. Methods include continuous purge, pressure control and concentration control. Continuous purge provides a constant flow of nitrogen and is probably the easiest method because a control device isn't required. However, nitrogen consumption is high. In pressure control blanketing, a valve allows the addition of nitrogen when the liquid level drops while a vent enables release of nitrogen when the liquid level rises. Concentration control blanketing relies on a feedback loop from an oxygen analyzer to the nitrogen generator to cycle the generator on or off. This method economizes the use of nitrogen because it shuts down the nitrogen supply until enough outside air infiltrates the tank headspace to raise the concentration of oxygen above the acceptable limit. NITROGEN SUPPLY OPTIONSPlants can obtain nitrogen supplies in several ways. These include receiving nitrogen as gas in high-pressure cylinders or as liquid in micro-bulk tanks (dewars) and bulk tanks. Nitrogen also can be generated on-site as a liquid by cryogenic means or as a gas on-demand by membranes or pressure swing adsorption (PSA). Following are the pluses, minuses and costs of each approach.Single cylinders hold about 240 ft3 (6.8 m3) of gas at an average cost of $15 to $35 per 100 ft3 (2.8 m3) and are the most expensive option. Cylinders come in a wide variety of volumes and fill pressures. Fill pressures typically are about 2,200 psi (152 bar). Usable cylinder volume is about 205 std. ft3 (5,800 L). Note that not all the gas in a cylinder can be used. Also, high output pressures will result in even more wasted gas. Cylinders can work well for low-flow applications such as welding that only need small amounts of gas, intermittently. However, cylinders can present safety issues, not only because of their bulk and weight. Should a cylinder be dropped, the canister can turn into a dangerous projectile.Dewars are vacuum-insulated vessels that have an integral, internal evaporator to convert the stored liquid nitrogen to gas. Important performance parameters for dewars include flow rate and duty cycle. The internal evaporator limits the ability to maintain a relatively high flow rate. Also, because of the internal evaporator, you must allow time for the liquid to reach a sufficient temperature to ensure complete evaporation. It's not uncommon for the evaporator to frost up and lose its ability to deliver gas at the desired flow rate. A typical dewar of liquid nitrogen holds around 3,500 ft3 (99 m3). Dewars must allow some liquid to evaporate to atmosphere to relieve internal pressure. However, dewars evaporate at different rates (older ones faster than newer ones) and lose gas volume over time. Therefore, capacity depends upon how long the dewar stands unused. Typical losses are 10% or more. The average cost to the user is $1.50 to $2.50 per 100 ft3.Figure 2. Carbon molecular sieves enable achieving 99.9%-pure nitrogen.
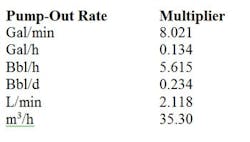
Bulk tanks containing liquid nitrogen generally are between 3,000 gal (11,356 L) and 11,000 gal (41,639 L) in size. Bulk vessels comprise a vacuum-insulated liquid storage tank housed in an outer carbon steel shell. Bulk tanks, like the smaller dewars, must leak nitrogen to prevent pressure buildup. A rating plate on the vessel exterior indicates tank capacity. A typical installation includes the bulk vessel and a finned heat exchanger or evaporator, both of which are located outdoors, as well as pressure regulation to the application and remote monitoring of the liquid level. The evaporator, which is located next to the tank on the delivery line, relies on the outside temperature to heat up the liquid nitrogen. The evaporators often frost up on humid days and become inefficient. Bulk tanks typically are rented. However, prior to a tank's installation, a plant will incur costs to pour a concrete slab to support it. The cost of nitrogen depends upon so-called "vaporization units" that relate to how much of the gas the user purchases annually. As of this writing, gas costs in the U.S. range from $0.35 to $0.90 per 100 ft3.Delivering nitrogen as a liquid in micro-bulk or bulk tanks especially suits low-temperature applications such as flash freezing. By comparison, other freezing methods are slow.Generating nitrogen on-site via a cryogenic plant usually is reserved for facilities such as large chemical complexes in remote areas. Air is liquefied by cooling to cryogenic temperatures, and split into argon, nitrogen and oxygen. The other gases and impurities are scrubbed out. An on-site cryogenic plant is best when a facility needs a high flow of nitrogen continuously and can utilize both the oxygen and nitrogen generated. An upside is that the facility doesn't pay for delivery and has almost a limitless supply of nitrogen. The production cost for nitrogen generally runs between $0.08 and $0.15 per 100 ft3. However, the approach requires a capital investment in a generator and possibly investment in a larger air compressor.At $0.15 or less per 100 ft3, on-demand nitrogen generators often represent the most-cost-effective option for applications such as chemical blanketing of flammable liquids that need a steady flow of nitrogen. Note that on-demand generators provide gas at ambient temperature and so can't be used for freezing or refrigerant-type applications requiring cold liquid nitrogen. The method frees the plant from relying on outside suppliers and the attendant problems such as long-term purchase contracts and commitments, inflexible delivery schedules, price increases and long procurement processes. Moreover, adding an additional work shift doesn't require investment in more equipment. In contrast, adding shifts with a bulk delivered gas will double or triple the volume of gas that must be bought, increase the number of deliveries, etc.An on-demand nitrogen generator also can help a plant meet sustainability objectives. Eliminating the need for another facility to generate nitrogen, store it and truck it reduces the entire carbon footprint required to supply nitrogen to the plant. Compared to third-party supplied bulk nitrogen, generation of 99.9%-pure nitrogen on-site with a PSA system uses 28% less energy. This lower demand for electricity translates into decreased creation of greenhouse gases. ON-DEMAND NITROGEN GENERATORSNitrogen gas generators typically are free standing, housed in a cabinet or skid mounted, depending upon the application. A plant only needs to connect a regular compressed air line to the inlet of the generator (after ensuring that a sufficient supply of compressed air is available) and attach the outlet to a nitrogen line. Standard features often include high-efficiency coalescing pre-filters with automatic drains and a sterile-grade after-filter.On-demand generators rely on either membrane or PSA technology. The choice largely depends upon the purity of nitrogen required for blanketing. Typically, applications such as fire prevention only need nitrogen of 95% to 98% purity and can use membrane generators. Applications such as the blanketing of oxygen-sensitive compounds, specialty chemicals and pharmaceuticals demand a higher purity stream and require use of PSA generators. As an example of how membrane nitrogen generators work, a Parker Balston generator (Figure 1) separates the compressed air into component gases by passing the air through proprietary semipermeable membranes consisting of bundles of hollow fibers. Each fiber has a circular cross-section and a uniform bore through its center. Compressed air is introduced into the bore of the fibers at one end of the membrane module. Oxygen, water vapor and other gases permeate the membrane fiber wall and are discharged at low pressure through a permeate port; the nitrogen remains within the membrane and flows at operating pressure through the outlet port. The exiting nitrogen gas stream is 95%–99+%-pure and very dry, with a dewpoint of at least -58°F (-50°C). Membrane units need no electricity to generate nitrogen, so they can be used in Class 1 explosion-proof environments without any concerns.In contrast, the Parker PSA nitrogen generator shown in Figure 2 uses high-efficiency pre-filtration to remove all contaminants down to 0.01 micron from the compressed air stream. The filters are followed by dual beds filled with carbon molecular sieves (CMS). In one bed at operating pressure, the CMS adsorb oxygen, carbon dioxide and water vapor. The other bed operating at low pressure releases the captured oxygen, carbon dioxide and water. Cycling the pressures in the CMS beds causes all contaminants to be captured and released while letting the nitrogen pass through. A final sterile-grade filter ensures removal of any microbial contamination. You can easily set purities with a flow control valve. For example, the DB-30 nitrogen system produces a flow of nitrogen as great as 1,530 std. ft3/h at 99.9% purity. The unit can achieve higher flow rates if gas of lower purity is acceptable. A built-in monitor measures the oxygen concentration of the nitrogen stream. The system requires a minimum feed pressure of 110 psi and can operate at pressures up to 140 psi. The resulting nitrogen has a dewpoint as low as -40°F (-40°C). CONSIDER ON-DEMAND NITROGENBoth membrane and PSA units produce nitrogen at precise purities, flow rates and pressures. A single nitrogen generator can meet your needs for shifts ranging from 8 to 24 hours.Generating your own nitrogen frees you from reliance on a third party and its terms and conditions. Moreover, compared to other options, on-demand generators provide added safety and significant cost savings as well as a sustainable approach to the supply of nitrogen.Table 1. Use the appropriate multiplier to determine std. ft3/h of air required. Source: Tyco.
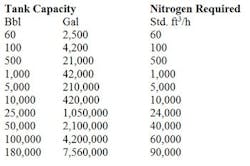
Table 2. Tank capacity determines the std. ft3/h of air required. Source: Tyco.
Note that in a pressurized system the makeup nitrogen demand might be fairly low if a user rarely dumps the tank. However, it's important to provide sufficient capacity to maintain the blanket during tank pump-out and changes in height due to temperature. In this case, size the generator to accommodate the pump-out speed. For example, a blanket provided by pressure affects the chemical in a different way than a blanket provided by continuous purge. In addition, a non-pressurized system also must accommodate the contraction and pump-out speed and maintain a blanket to keep up with leakage.DAVID J. CONNAUGHTON is product marketing manager, industrial gas generators, global development, for the Filtration and Separation Division of Parker Hannifin Corporation, Haverill, Mass. E-mail him at [email protected].