Catalyst Boosts Butadiene Production
Researchers at North Carolina State University (NCSU), Raleigh, N.C., have developed a new catalyst that improves n-butane-to-butadiene conversion efficiency, and could lead to increased butadiene production to meet growing demand.
Existing conversion techniques create unwanted byproduct, or convert only a small fraction of the butane in each run through the reactor, note the researchers.
“This is an expensive process in terms of both energy and money,” says Fanxing Li, a professor of chemical and biomolecular engineering at NCSU. “Because after every pass through the chemical reactor, you have to separate the butadiene and byproducts from the butane — which takes a lot of energy — and run the butane through the reactor again.”
Because of this, few plants produce butadiene. According to the researchers, much of the butadiene used in manufacturing comes from plants where butadiene is a byproduct of other reactions.
“That’s a problem, because the demand for butadiene far outstrips the available supply,” Li emphasizes.
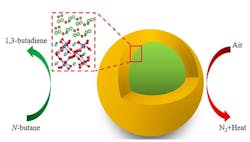
So, to make butadiene production facilities more commercially viable, the researchers engineered a lithium bromide (LiBr) shell catalyst surrounding a core of lanthanum strontium ferrite (Figure 1). Using a modular reactor, an oxidative dehydrogenation reaction run at 450°C to 500°C converts more butane into butadiene with each pass through the reactor than achieved with previous catalysts, they report.
“We were able to convert up to 42.5% of the butane into butadiene in a single pass. The previous best performance we could find was around 30%. We may be able to push it up to the 50% range, but we believe it is more important to decrease the COx yield to, say <5%,” says Li.
In an article in Science Advances, the team writes, “Further investigations on the effect of long-term LiBr loss and strategies for on-stream replenishment of the LiBr promoter would be important from a practical standpoint.” Such plans to experiment will take place sometime within the next 6 months, notes Li.
“This is a big first step,” he adds, “but we view it as a proof of concept — we think we can still do a lot more to improve the selectivity of this process. We believe the main drawback of our redox catalyst is its relatively high selectivity towards CO2/CO (COx). We believe that COx formation can be minimized by further improving the promoter and promoter oxide combinations and we have a few ideas about that as follow up studies,” he explains.
The core-shell-structured redox catalyst reportedly offers adequate mechanical stability in packed bed reactors. “We have not quantified its attrition resistance for fluidized-bed operations, but our preferred reactor design is packed bed,” elaborates Li. Furthermore, “Based on our previous testing, the redox catalyst performance should not be affected by other light hydrocarbon contaminants in industrial n-butane,” he adds.
Further work is needed before scaleup can begin. “We believe it is feasible to use industrially proven methods, such as extrusion and wet impregnation, to scale up the production of the catalyst. But further improvement of the catalyst performance, e.g., reducing COx selectivity and yield, would be important before we scale up the production of the catalyst, stresses Li.
“We’re open to partnerships to further explore the potential of this work,” he concludes.