Chemical makers seeking to improve performance and efficiency should consider adopting ISO 9001:2015. The standard sets criteria for a quality management system (QMS) and can enable an organization to assess its ability to meet customer and regulatory requirements.
Organizations currently registered to the ISO 9001:2008 QMS standard will have until September 2018 to transition to the ISO 9001:2015 version or acquire ISO 9001 registration and certification under the updated standard.
Why go through this process? Implementing the ISO 9001:2015 standard can lower operating costs and improve output quality, ultimately increasing the level of customer satisfaction.
[callToAction ]
There’s a key relationship between quality of materials and quality of final product and this particular ISO standard helps ensure quality of raw material input, therefore strengthening the quality of the final product. It also can positively impact leadership, management and supplier relationships. Here are some fundamentals to know about ISO 9001:2015:
1. The standard empowers an organization to structure its QMS to align with its specific context in the global market and its vision. Specifically, the updated standard states the organization doesn’t need a quality manual as long as it has a well-developed quality policy suited to its particular requirements and this is deployed in such a manner that all employees are clearly aware of their role in fulfilling the organization’s vision.
2. A “quality assurance management representative” is no longer required; this implies the organization must hold all individuals in the chain of command formally accountable for their contributions to the key performance indicators (KPIs) that are aimed at achieving its vision. For those focused on measuring customer satisfaction, ISO 9001:2015 states that for every process (manufacturing or otherwise) the organization must: a) integrate its business processes with the ISO 9001:2015 standard’s processes, thus leaving nothing as a standalone silo; and b) ensure that at least one KPI (also referred to as a metric by some organizations) for each process objectively shows the performance of that process and which can be controlled by the employees involved in executing that process. Of course, the organization has full freedom to decide which KPIs must be established as well as the frequency of their updates, review and follow-up.
3. It strongly encourages the leaders in the organization to use a risk-management-based and proactive approach to problem solving and, more importantly, problem prevention using the Plan-Do-Check-Act cycle. The standard doesn’t specify the use of any specific tools from the “Lean” body of knowledge but a well-trained and experienced Lean practitioner will know which tool to use based upon the organization’s level of maturity in its Lean journey.
4. The standard allows an organization to decide precisely which documents are necessary to maximize its QMS’ effectiveness. There no longer is a need for non-value-added documents to exist simply for the sake of complying with certain sections of a standard that in many cases don’t apply to the organization. This is one of the most important steps forward in any organization’s lean journey and ISO 9001:2015 formally enables an organization to take this step with confidence. The obvious caveat to this potentially wonderful enhancement is that an organization shouldn’t be too zealous in getting rid of any existing data, documentation and records if they have proven valuable recently or even in the past.
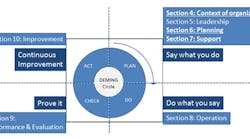
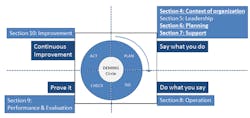
Figure 1 summarizes the key changes between ISO 9001:2015 and its predecessor. The solid, underlined sections denote where the enhancements have been made.
With that brief introduction, here are the possible improvements for those chemical companies that wish to transition to this standard or register to it for the first time.
Figure 1. The 2015 version of ISO 9001 includes important revisions in several sections.
Management Structure
Not requiring a quality assurance manager lifts a significant financial burden off any company. However, that means every individual in the organization must learn to effectively execute the responsibility traditionally left to the quality assurance manager, i.e., implementing, using and managing a well-developed QMS. Making data-driven go/no-go decisions aimed at maximizing customer satisfaction or employees’ safety without a quality assurance manager can be a particularly tricky and, sometimes, a career-ending task. This is especially true at many chemical makers, where downtime while a decision is being made may cost thousands of dollars every hour due to the continuous nature and sheer volume plus cost of material associated with their processes. Therefore, all individuals in the organization must control the portions of the QMS relevant to their job description; each person must have a clear, objective basis with which to effectively resolve such demanding situations once and for all.
Consider, for example, a batch of expensive plastic film that has been quarantined and potentially may be rejected because the laboratory quality technician has determined (using a calibrated, reliable densitometer) that its density falls below the lower specification limit by two units, which is a 2.5% deviation relative to that limit.
Under the ISO 9001:2008 standard, the quality management representative, much to the chagrin of plant management, would make the final go/no-go decision.
In contrast, under ISO 9001:2015, plant management can assign decision-making responsibility to any of its members. However, the standard does impose a condition — that the individual charged with this onerous task is adept at using a simple, efficient and standardized decision-making flowchart. This would describe clearly in objective terms whether the product should be rejected or shipped — with the satisfaction of the customer being the foremost goal.
This flowchart can be developed and systematically modified by the same individual using input from the customer as to whether a 2.5% deviation relative to the specification limit has practical significance given the film’s intended application.
Of course, some might argue that a member of plant management always will err on the side of profitability and productivity by sending what ought to be rejected product to the customer.
Indeed, the person can do so and still comply with the ISO 9001:2008 and even the ISO 9001:2015 requirements. However, most customers can switch to alternative suppliers once they realize the product’s quality no longer is up to par or its cost isn’t competitive anymore. Manufacturers that don’t put their customers’ interest first won’t last, especially these days!
Risk Management
Fundamentally, the classic definition of risk management is a data-driven identification and prioritization of risks followed by application of available resources to minimize, monitor and then control the probability and impact of unfortunate events or to maximize the positive impact of identified opportunities. Clearly, customer satisfaction — a key goal of any organization — can’t be sustained by accident. So, to ensure long-term success, a manufacturer must use risk management. While regulations and good engineering practices have spurred chemical makers to adopt risk management for process safety, the broader use of the approach lags (not just in the chemical industry but throughout manufacturing in general). The simplest risk-management tool any organization can learn and successfully apply in almost every situation is the Plan-Do-Check-Act cycle, which is emphasized in the new standard.
Let’s consider an example. A chemical company determines that overall equipment efficiency is a KPI of productivity in the bottleneck process in a high-volume facility. After all, if the organization can’t meet customer “takt time” (i.e., the rate of production needed to satisfy its demand), the customer will turn to a competitor who can meet it — and that spells financial disaster.
As a result of this determination, the company decides to purchase and install a software and hardware package for quantifying the different time-utilization packets for this bottleneck process.
The chemical maker can use a simple risk-assessment technique (e.g., failure modes and effects analysis) to identify what can go wrong with this purchase and installation process. That then leads to proactive risk mitigation by resolving the possible root causes using a simple Lean technique such as “five whys,” thereby making the return on investment more reliable for upper management.
Again, ISO 9001:2015 gives the organization full freedom to use whichever risk-management and problem-solving tools it wishes; the standard provides no mandate in that regard. For the sake of simplicity and efficiency, the organization should adopt the easiest formats and approaches; this will foster its workforce’s participation and confidence in these efforts. That’s the Lean way.
Personal Accountability
The one factor that can make an organization a master in its market is having every employee think and act like a quality assurance manager or, more precisely, a continuous improvement expert. GE offers an excellent example of how to do this. The company provides extensive Lean and Six-Sigma knowledge to each level of its hierarchy and then holds people accountable for using that knowledge on a daily basis. That leads to the creation at every level of the organization of true self-driven leaders, who no longer depend upon a quality assurance manager to save the day.
For example, a chemical plant determines 12 KPIs as relevant to objectively gauging the health of its business — two in each of the following six categories: safety; housekeeping; delivery; quality and customer satisfaction; employee training; and productivity.
The plant manager, who is the highest level of management on site, creates a shop-floor management structure at the site level, the department level and even at the shift level using deviation management as the foundation. This ensures that even the smallest deviation (e.g., only 1,000 kilograms of product manufactured in 8 hours compared to the customer takt time of 1,006 kilograms of product per 8 hours) is dealt with in a timely manner to prevent this from becoming a larger deviation.
The plant manager leads via action by ensuring the daily production meetings are carried out at the shop-floor management boards. Using the “go out and look” approach in conjunction with timely deviation management, a simple and effective problem-solving approach brings KPIs back on track. The problem-solving approach adopted will depend upon the complexity of the root causes associated with the deviation and could range from the use of an 8D (eight disciplines) format to a cross-functional DMAIC (design, measure, analyze, improve, control) project.
With ISO 9001:2015, a management representative isn’t required to take charge of this group of tasks, so it’s plant management’s responsibility to use this simple but incredibly effective Plan-Do-Check-Act cycle to identify and resolve even the smallest deviations in a timely and cost-effective manner before they become too big to handle.
Adopt ISO 9001:2015
Incorporating the updated standard into an organization’s culture will make implementing all the changes outlined here relatively easy. Even more important, the revisions now will require leaders in the chemical industry to use the standard as the powerful management tool it was designed to be. For more information on ISO 9001:2015, visit the American Society for Quality website.
GANAPATHY SUBRAHMANIAM is an operational excellence manager, quality assurance manager and HSE manager for Voith GmbH at its Voith Paper Fabrics manufacturing facility in Shreveport, LA. E-mail him at [email protected].