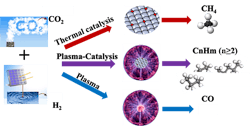
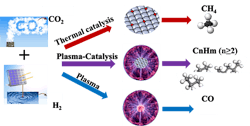
Plasma Eases Carbon Dioxide Conversion
Carbon dioxide conversion through catalysis normally requires a great deal of energy with multiple steps, high temperature (roughly 400–750°F) and high pressure (150–600 lb/in2). Now, a team of researchers have developed a one-step, plasma-enabled catalytic process to convert CO2 into higher hydrocarbons. It operates at low temperature (75°F) and pressure (15 lb/in2) using a dielectric barrier discharge (DBD) packed-bed plasma reactor. In addition, tests of the process demonstrated a higher hydrocarbons selectivity of 46% at a CO2 conversion rate of 74%, they report. The researchers used a conventional aluminum oxide-supported Fisher-Tropsch catalyst for the process.
The researchers — from Pennsylvania State University (Penn State), University Park, Penn.; the Chinese University of Hong Kong; and Sichuan University, Chengdu, Sichuan, China — published their findings in a recent article in Green Chemistry.
High-energy electrons within the plasma phase activate both CO2 and H2 molecules in the gas phase through excitation and dissociation. This happens without surface adsorption and activation as required for traditional thermal catalysis, so the reaction operates at low temperature, notes Xiaoxing Wang, associate research professor at the Penn State EMS Energy Institute.
Figure 1. Conversion method operates at low temperature and pressure, and can be powered with renewable energy.
“Besides, catalysts, catalyst-bed configuration, and reaction conditions such as gas space velocity, CO2/H2 ratio, plasma power, etc., can also greatly influence the final selectivity of C2+ hydrocarbons. Our target is to attain over 80% of selectivity for C2+ hydrocarbons,” Wang adds.
“The electricity generated from renewable energy easily can be adapted to the plasma-catalysis process. The catalyst deactivation due to high temperature sintering observed in conventional thermal catalysis largely can be avoided in the plasma-catalysis process. Furthermore, plasma can enhance catalyst dispersion and suppress metal agglomeration,” claims Wang.
Compared with the conventional process, the plasma-enabled catalysis offers more flexibility in operating parameters and feed gases, greater energy efficiency, and less sensitivity to poisoning, say the researchers.
“The plasma discharge zone can expand to cover the entire volume of the reactor for the reaction. In addition, the process doesn’t need sophisticated or costly devices and materials, especially for the DBD plasma. Those advantages make the process promising for scaling up,” believes Wang. “To scale the plasma-catalytic reactor up to a size that competes with the well-established large-scale commercial processes would require a significant advancement in plasma-catalysis science and technology,” he admits.
“By changing the catalyst-bed configuration, we successfully managed to obtain a high yield of C2+ hydrocarbons and found the developed process likely involves two tandem conversions including plasma-enhanced CO2 hydrogenation to CH4 over Co catalyst, followed by plasma-induced methane coupling to C2+ hydrocarbons. Both reactions determine the final selectivity of C2+ hydrocarbons. Recently, we examined other catalysts including iron and nickel catalysts. Interestingly, we only obtained >99% CO over the Fe catalyst, while CH4 was the main product over the Ni catalyst. Without CH4 formation, C2+ hydrocarbons barely formed. The Ni catalyst is even more effective than the Co catalyst for CH4 formation. Now, the key is to find a catalyst for the second conversion, the plasma-induced methane coupling reaction, to further improve C2+ production and to control the distribution of C2+ hydrocarbons such as C2-C4 olefins, C5-C12 gasoline, C12-C20 diesel,” explains Wang.
“The plasma-activated catalytic process is promising for CO2 conversion,” emphasizes Wang. “It can be applied to other CO2 conversion reactions such as CO2 splitting, dry methane reforming, etc. The investment and operating costs are relatively low. It [also] can be applied in a modular setting and scaled up linearly with the plant output. It can be easily combined with renewable electricity, thus is suitable for converting intermittent renewable energy into base chemicals and/or fuels for peak shaving and grid stabilization,” he says.