Catalyst Pushes The Edge
A new catalyst for dry reforming of methane could provide an economically feasible route for large-scale use of carbon dioxide as a feedstock and, thus, an important tool for dealing with greenhouse gas emissions, hope researchers in Korea. Their catalyst, by concentrating nickel nanoparticles on the support’s edges, overcomes cost and technical problems posed by current catalysts for dry reforming. Those catalysts contain rare and expensive metals such as platinum and rhodium and are prone to deactivation through a combination of coking and sintering.
The attraction of nickel as a more economical catalyst isn’t new. However, build-up of byproducts on its surface have caused problems.
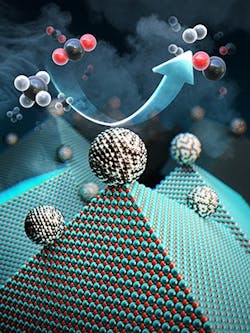
Figure 1. Unique structure overcomes coking and sintering problems that have afflicted other catalysts. Source: Cafer T. Yavuz, KAIST.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” says Cafer T. Yavuz, associate professor of chemical and biomolecular engineering and of chemistry at Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea.
Yavuz and his co-workers have developed a technique they believe overcomes all these problems. Details on their method, dubbed nanocatalysts on single crystal edges (NOSCE), appear in a recent issue of Science.
NOSCE involves using highly crystalline fumed magnesium oxide to support molybdenum-doped nickel nanoparticle catalysts (NiMoCats). On heating, the nanoparticles migrate to form larger, highly stable nanoparticles on the oxide surfaces’ edges.
In tests, the catalyst has operated continuously for more than 850 hours with a reactive gas flow of 60 L/mass of catalyst/hr with no detectable coking.
Synchrotron studies showed that sintering did not occur and also revealed the complex movement of nanoparticles and magnesium oxide crystals during catalyst activation.
“It took us almost a year to understand the underlying mechanism,” notes Youngdong Song, a graduate student at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The next phase in the work involves pilot plant testing with stable catalyst pellets, states Yavuz. “The main challenge at the moment is pellet stability. Our initial runs with commercial binders showed great activity but low mechanical stability. We’re now improving the procedures for better pelletization,” he explains.
The group also looks to expand the application of NiMoCat to other reactions. “As anyone would guess, we’re already into higher hydrocarbons and mixed (steam and dry) reforming procedures. But, in principle, any nickel-catalyzed reaction could benefit from our design,” he adds.
Saudi Aramco has been funding the work so far through the Saudi Aramco-KAIST CO2 management center.
“Now, we’ll work with known catalyst companies to make the process plant scale,” concludes Yavuz.