Carbon Utilization Gets A Boost
A recently developed process that creates carbon-nitrogen bonds during electrochemical carbon monoxide reduction offers a new route for producing a variety of acetamides, say researchers from the University of Delaware (UD), Newark, Del., along with collaborators from the California Institute of Technology (Caltech), Pasadena, Calif.; Nanjing University, Nanjing, China; and Soochow University, Suzhou, China. The process could further advance carbon capture and utilization (CCU) and extend its promise to the pharmaceutical industry among others, they note.
“This has significant impact down the road, I think, to partially address carbon dioxide emission issues,” says Feng Jiao, an associate professor of chemical and biomolecular engineering at UD, and the associate director for UD’s Center for Catalytic Science and Technology (CCST). “Now we can actually utilize it as carbon feedstock to produce high-value chemicals,” he adds.
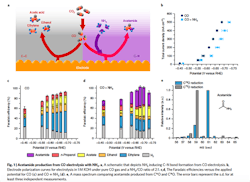
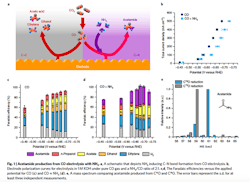
The process involves feeding an electrochemical flow reactor with both carbon monoxide and ammonia. The ammonia’s nitrogen in the presence of a copper catalyst reacts with carbon at the electrode/electrolyte interface at ambient conditions to form carbon-nitrogen (CN) bonds. “This actually provides a unique way to build large molecules which contain nitrogen from simple carbon and nitrogen species,” notes Jiao.
“In the field of electrochemical carbon dioxide or carbon monoxide reduction, only four major products are reported, including ethylene, ethanol, acetate and n-propanol. While these commodity chemicals have values, their market prices are not that high. Our previous study of techno-economic analysis of carbon dioxide electrolysis technologies suggested we target high-value chemicals because of the significant cost of electricity in the United States. Chemicals for pharmaceutical industries often contain heteroatoms, such as nitrogen and sulfur. This motivates us to look into ways to build these heteroatoms into the products that we can produce electrochemically from abundant sources such as CO2 and ammonia,” says Jiao.
Figure 1. Schematic depicts ammonia inducing carbon-nitrogen bond formation from CO electrolysis. Source: Feng Jiao, University of Delaware.
“The important insights obtained from this work include the identification of ketene as a key intermediate in copper-catalyzed carbon monoxide reduction reaction and the possibility to form carbon-heteroatom bonds by co-feeding a nucleophilic agent with carbon monoxide. The collaboration with Caltech further enabled us to establish a detailed reaction mechanism, a critical knowledge towards rational design of advanced catalysts. For example, we can tune the property of the catalyst and make it more favorable for one reaction pathway than the other. Such a catalyst design requires us to know which intermediate holds the key for product selectivity,” he adds.
The team performed an 8-hr electrolysis test to determine the stability of the copper catalyst and susceptibility to poisoning. Results showed no loss of catalyst activity and selectivity. “We also examined the structural stability of the catalyst … Of course, more studies will be required to investigate catalyst stability at a much longer time scale for practical applications,” admits Jiao. “We also haven’t tested other potential contaminants that could be present in common carbon monoxide or carbon dioxide sources. This will be something for future studies,” he adds.
The researchers reported in a paper in Nature Chemistry acetamide selectivity of nearly 40%. “The work we just published is the first demonstration of CN-bond formation in electro-reduction of carbon monoxide in the presence of ammonia (or amine). There is plenty room to improve the selectivity, which is important to minimize product separation costs,” notes Jiao.
The team will next examine possibilities to form other products using this process. “We are also interested in investigating the role of the catalyst in the CN-bond formation, which is an unexplored area at the moment,” says Jiao.
The researchers have filed an international patent through the UD based on this discovery. Jiao and Gregory Hutchings, a former graduate student, have developed a startup company to potentially commercialize the technology and are looking for industrial partners.