A new switchable dual-function material (DFM) developed by researchers at the U.K. University of Surrey captures and converts CO2 into green fuels or useful industrial chemicals.
The DFM, NiRuNa/CeAl, consists of nanoparticles of a bimetallic alloy in combination with a dispersed Na-based adsorbent.
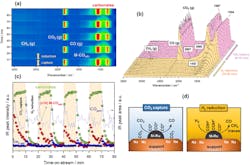
Using a technique called operando-DRIFTS-MS, the researchers observed the capture of CO2 at the DFM surface and its conversion via three different reactions: CO2 methanation; reverse water-gas shift; and dry reforming of methane.
“We now have a clearer understanding of how switchable DFMs are able to perform a multitude of reactions directly from captured CO2, which will help us improve the performance of these materials even more via rational design,” says Melis Duyar, chemical and process engineering professor and study lead.
One challenge is to overcome the coking observed at the Na-Ni/Ru interface during methane cracking.
“On the process side, we have seen that methane cracking occurs readily after all the adsorbed CO2 has been consumed via the DFM reaction. Therefore, introducing an exact pulse of methane that is no more than is needed to convert all the CO2 can offer a solution,” she explains.
“Designing materials with uniform interfacial sites to maximize adsorbent-catalyst interaction is a key scientific challenge for minimizing side reactions as well as preventing the desorption of unreacted CO2,” she adds.
Next, the team hopes to develop computational tools both to speed up DFM discovery and to optimize the process involved. Advanced synthesis techniques will be investigated to increase CO2 capture capacity and conversion, lower the temperature of operation, and minimize side reactions such as methane cracking.
“What is really powerful about this kind of study is that it provides valuable insight for designing the next generation of DFMs, meaning we can rationalize our material design decisions using fundamental understanding of the surface phenomena,” Duyar says.
The researchers are currently collaborating with industrial partners on an upcoming project aiming to validate this technology using real CO2 emissions.
“We believe this kind of collaboration can help us bring our technology to the field, accelerating the technology transfer to where it is needed. We have also filed a patent application that can allow companies to license our technology and use it to meet their carbon capture and chemicals production needs,” Duyar concludes.