As a snapshot of the most recent catalyst research reveals, novel visualization techniques are giving unprecedented insights into how they function, while tackling the problem of plastic waste and harnessing the power of sun remain priorities.
In one such visualization development, for example, scientists at the Institute for Basic Science in Daejeon, South Korea, have observed the long-postulated intermediate in catalytic amination reactions.
Known as a transition metal-nitrenoid, it is crucial in converting hydrocarbons into the amides used in chemical and pharmaceutical applications.
The scientists, based in the institute’s Center for Catalytic Hydrocarbon Functionalizations, used x-ray photocrystallography with a novel chromophoric rhodium/ligand complex to track chemical reactions in solid-state.
Then, carrying out photoinduced single crystal x-ray diffraction analysis using synchrotron radiation on the isolable complex formed, the scientists managed to reveal the structure and properties of the rhodium-acylnitrenoid intermediate for the first time.
Moreover, this study was designed also to achieve crystallographic monitoring of rhodium-acylnitrene transfer toward an external nucleophile, all in the solid phase, which provides complete mechanistic snapshots of the nitrenoid transfer process.
Chang Sukbok, director of the Institute for Basic Science, believes the work, as explained in Science, provides clues for the design of highly reactive and selective catalysts that could be useful across various industries. “Possibly even contributing to the development of a ‘universal catalyst,’” he added.
Meanwhile scientists from Uppsala University in Sweden, the Paul Scherrer Institute in Switzerland, Stockholm University, Hamburg University and the European x-ray free-electron laser facility have used x-rays to visualize a single carbon-hydrogen bond break in an alkane molecule.
Researchers performed the time-resolved x-ray absorption experiments, described in a another issue of Science, on a rhodium catalyst immersed in a dense octane solution.
Using beamlines at two of the world’s most powerful sources of x-ray flashes, the reaction was followed from beginning to end. These revealed the initial light-induced activation of the catalyst within 400 femtoseconds to the final C-H bond breaking after 14 nanoseconds.
Using advanced quantum-chemical calculations, the scientists noted the importance of the amount of electronic charge flowing from the metal catalyst to the C-H group and vice versa. When it flows from the metal onto the C-H bond, it glues the two chemical groups together. A charge in the opposite direction acts like scissors, eventually breaking the C and H atoms apart.
In the future, the researchers want to learn how to direct the flow of electrons to help develop better catalysts for the chemical industry.
In another imaging development, scientists at the Vienna University of Technology, or TU Wien, in Austria used correlative photoemission electron microscopy and scanning photoemission electron microscopy to show that seemingly “simple” catalytic systems are more complex than expected.
Investigating the catalytic behavior of rhodium particles supported by three different materials – rhodium, gold and zirconium dioxide – during hydrogen oxidation, they found that it is not only the size of the employed metal particles, or the chemical nature of the support material that define the catalytic properties.
Even within a single metal particle, different scenarios can prevail on the micrometer scale.
“Atoms of the support material can migrate onto or into the particles or even form surface alloys,” states Günther Rupprechter, professor in the Institute of Materials Chemistry at TU Wien. “At some point, there is even no clear boundary anymore, but rather a continuous transition between catalyst particle and support material. It is crucial to consider this fact – because it also affects the chemical activity.”
The TU Wien researchers complemented the experimental observations with micro-kinetic simulations based on variations of hydrogen adsorption and oxygen binding. The results, published in ACS Catalysis, demonstrate how correlative in-situ surface microscopy enables linking of the local structure, composition and catalytic performance in a way that both explains and correctly predicts the behavior of the different catalysts.
The TU Wien scientists now plan to apply their new knowledge to more complex catalytic processes.
Probing Polymer Recycling
Researchers at the University of California, Santa Barbara have developed a bifunctional tandem catalytic method for upcycling polyethylene (PE) to surfactant-range alkylaromatics.The work, featured in Chem, builds on an earlier catalytic method they developed to break strong carbon-carbon bonds and then rearrange the molecular chains into alkylaromatic rings.
“While effective, the original process, based on a platinum-on-alumina catalyst, was slow, and its yield of alkylaromatic molecules was low, says chemical engineering professor Susannah Scott, who holds UCSB’s Mellichamp chair in sustainable catalytic processing. “What we’ve done in this paper is show how to do it much better.”
This involved supporting platinum nanoparticles on a variety of acidic solids to investigate the effect of acidity on both the rate of carbon-carbon bond cleavage and the selectivity to alkylaromatic products.
Key to their method is increasing the acidity of the original alumina catalyst via the addition of chlorine or fluorine. With the added acid sites, the team was able to boost the speed of PE conversion and generate a higher fraction of alkylaromatics in the C16-C22 molecular weight range used as surfactants and lubricants.
In addition, their one-pot process operates at moderate temperatures, requiring a low energy input. While the method originally took 24 hours to turn PE into alkylaromatic molecules, the improved process can complete the task within a couple of hours, increasing the amount of plastic that can be converted in a reasonably-sized reactor (Figure 1).
Scott notes that the process could become a viable commercial process with further improvements. For example, strongly acidic catalysts produce more low-value gases, polyaromatics and coke during PE upcycling. Also, the impact or inorganic fillers, organic additives and other polymers in post-consumer PE waste need to be minimized. The surfactant properties of the PE-derived alkylaromatics upon sulfonation need investigating too.
“On the basis of the knowledge acquired, we are developing other catalyst models that are more efficient and more persistent in ambient conditions, making them more suitable for industrial applications,” she explains.
One challenge here is overcoming the highly air sensitive nature of the catalysts, which is one of the most significant limiting factors for this system.
“We are currently working on improving this point, by modifying the catalyst structure and inventing a new technique for keeping the catalysts safely in the air,” Scott says.
The UCSB research team is developing this catalytic process with global silicon company Elkem Silicones, which is looking to industrialize the system, she says.
A new technique developed by chemical engineers at the University of Wisconsin–Madison converts low-value plastic waste into high-value products. They use several existing chemical processes to achieve their results.
First up is pyrolysis. The resulting oil contains large amounts of olefins, which are
recovered and used in a much less energy-intensive homogenous hydroformylation catalysis process. This converts olefins into aldehydes, which can then be further reduced into important industrial alcohols.
“We’re really excited about the implications of this technology,” says George Huber, professor of chemical and biological engineering who led the work and who also directs the Department of Energy-funded Center for the Chemical Upcycling of Waste Plastics. “It’s a platform technology to upgrade plastic waste using hydroformylation chemistry,” he adds.
Postdoctoral research associate Houqian Li believes the technique could provide a more sustainable and lucrative way for the industry to deal with pyrolysis oil, which is often run through steam crackers to produce low-value compounds (Figure 2).“Currently, these companies don’t have a really good approach to upgrade the pyrolysis oil,” says Li. “In this case, we can get high-value alcohols worth $1,200 to $6,000 per ton from waste plastics, which are only worth about $100 per ton. In addition, this process uses existing technology and techniques. It’s relatively easy to scale up.”
The research has spawned many follow-up opportunities, says Li. “We are producing samples for testing as surfactants to compare their properties with those of the surfactants generated from fossil feedstocks,” he explains. “We also plan to explore the approaches to tune the carbon distribution of the products generated from this route.”
This testing will involve different waste plastic feedstocks to adjust the branching properties of the final products, varying the reaction conditions to influence both carbon distribution and the functionalities of the chemicals produced, plus manipulating the parameters and gases used in the pyrolysis step to make this step more selective and energy efficient.
In China, a research team led by professor Zeng Jie from the University of Science and Technology of China in Anhui, has developed a solvent- and hydrogen-free method to upcycle high-density polyethylene (HDPE) plastics.
The findings, described in a Nature Nanotechnology article, involve a novel dehydroaromatization and hydrogenolysis tandem strategy.
The team focused on catalytic reforming of short-chain gasoline fractions to obtain higher-value cyclic hydrocarbons, which generates hydrogen, and the hydrocracking of heavy oils to produce short-chain hydrocarbons, which consumes hydrogen.
Described as a “hydrogen breathing” strategy, they developed a molecular sieve-loaded metallic ruthenium catalyst that facilitates the dehydrogenation of the plastic into cyclic hydrocarbons. This "breathes out" hydrogen in the process. Simultaneously, the plastic "breathes in" the released hydrogen and undergoes cracking, transforming into short-chain hydrocarbons.
The research team then explored the upcycling reaction pathways of HDPE plastics with different sieve loadings and pore sizes.
The results show that the molecular sieve has a moderate pore size, which not only avoids the formation of thick cyclic aromatic hydrocarbons and carbon deposits, but also ensures the smooth desorption of cyclic hydrocarbons, thus guaranteeing the continuity and stability of the catalytic reaction.
Shining a Light on Photocatalysis
Scientists at the Tokyo Institute of Technology in Japan have developed a tin-based, high-performance, precious-metal free, single-component photocatalyst for visible-light-driven reduction of carbon dioxide to formate. It is a metal-organic framework (MOF) called KGF-10, as described in an issue of Angewandte Chemie.
"Most high-performance carbon dioxide reduction photocatalysts driven by visible light rely on rare, precious metals as principal components,” explains research lead professor Kazuhiko Maeda. “Furthermore, integrating the functions of light absorption and catalysis into a single molecular unit made up of abundant metals has remained a long-standing challenge. Hence, tin was the ideal candidate as it can overcome both challenges.”
After analyzing the new material and finding that it showed moderate carbon dioxide adsorption ability and absorbed visible light wavelengths, the scientists used it for catalyzing the reduction of the gas in the presence of visible light.
They found that KGF-10 successfully reduced carbon dioxide into formate with 99% selectivity without needing any additional photosensitizer or catalyst.
Maeda believes these properties will open new avenues for KGF-10 as a photocatalyst in many other reactions, too.
In Hong Kong, a joint research team led by scientists from the City University (CityU) have developed a stable artificial photocatalytic system that achieves 15% solar-to-fuel efficiency when converting carbon dioxide into methane.
To achieve this efficiency, which tops that of natural photosynthesis, the team had to overcome the tendency of artificial photocatalytic systems to degrade in water.
So they used a supramolecular assembly approach that mimics the structure of a purple bacteria’s light-harvesting pigment containing chromatophore cells.
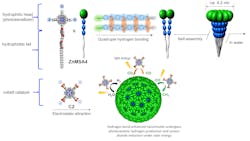
Their highly stable spherical chromatophore nanomicelle system has a hydrophilic head which functions as a photosensitizer to absorb sunlight, and a hydrophobic tail that induces self-assembly (Figure 3).Ultrafast spectroscopy, discussed in a Nature Catalysis article, revealed that the electrostatic interaction between the photosensitizer and the cobalt catalyst used in the reaction, and the strong light-harvesting antenna effect of the nanomicelle improved the photocatalytic process.
The current research has several limitations, so the team will be working to simplify the synthetic route, understand the electric field interactions, and select other molecular complexes to improve energy conversion efficiency, says Ye Ruquan, associate professor in the department of chemistry at CityU.
Other potential catalytic materials are also gaining attention. “We are currently working with several materials, such as metal-based nanomaterials, molecular interfaces and investigating their applications in other reactions, such as carbon dioxide-to-methanol conversion, water splitting and nitrate reduction,” he explains.
The researchers believe that the simple self-assembly system and use of abundant, cheap metal elements will herald the development of new artificial photocatalytic systems.