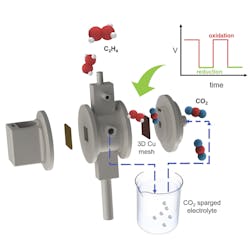
The cell achieved a Faradaic efficiency of nearly 58%, producing gaseous C2H4 with a purity of 52 wt. % and no CO2 in the product stream, report the researchers.
Two Spectrolab XTJ triple-junction solar cells used in tandem with the electrochemical cell converted 10% of the energy from the solar panels directly to the carbon product output. This is well above the state-of-the-art standard of 2%, the researchers write in a recent article in Cell Reports Physical Science.
In addition to C2H4, the UIC researchers were able to make other carbon-rich products useful to industry with their electrolysis approach.
The process can convert up to six metric tons of CO2 into one metric ton of C2H4, recycling almost all the CO2 captured, says research lead and UIC assistant professor of chemical engineering Meenesh Singh. Because the system runs on electricity, the use of renewable energy can make the process carbon negative, he adds.
This approach surpasses the net-zero carbon goal of other carbon capture and conversion technologies by actually reducing the total CO2 output from industry, emphasizes Singh.
“It’s a net negative. For every one ton of ethylene produced, you’re taking six tons of carbon dioxide from point sources that otherwise would be released to the atmosphere,” he explains.
Scaling-up the process poses engineering challenges, including increasing the energy efficiency of the process beyond 60% while improving both product separation and collection efficiencies, Singh admits.
For now, the team is focused on scaling-up integrated reactors that can capture CO2 directly from flue gas and covert it to produce 1–10 kg/d of C2H4.
The work is garnering industrial support, with Braskem America, Philadelphia, which operates five polypropylene plants in the United States, the principal sponsor. The company’s level of investment remains confidential.