Electrolyzer Paves Way for Greener Chemical Manufacturing
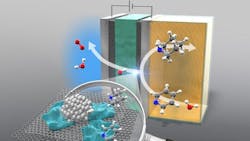
Researchers at Yokohama National University have developed a method for the hydrogenation of cyclic amines, particularly pyridines, using an anion-exchange membrane (AEM) electrolyzer. This approach aims to reduce the environmental impact of chemical manufacturing, especially in the production of fine chemicals and pharmaceuticals.
Traditional hydrogenation methods rely on hydrogen gas derived from methane steam reforming, contributing significantly to global CO2 emissions and energy consumption. The new AEM electrolyzer operates at ambient temperature and pressure, splitting water into atomic hydrogen and oxygen. This atomic hydrogen is then used for the hydrogenation process.
The process achieves a 78% yield at scale, demonstrating its scalability potential. However, challenges such as increasing cell voltage during electrolysis may need to be addressed through improved AEM design or AEMs specifically tailored for organic electrosynthesis.
“The method offers significant potential for industrial-scale applications in pharmaceuticals and fine chemicals, contributing to the reduction of carbon emissions and advancing sustainable chemistry,” said Naoki Shida, first author of the study and researcher at Yokohama National University, in a news release.
This electrocatalytic hydrogenation method represents a promising step toward more sustainable chemical synthesis, aligning with the industry's goals of reducing environmental impact while maintaining efficiency in the production of essential compounds.