Product stewardship is becoming a mission-critical concern for corporate executives in the chemical industry. The quest now has taken on a new dimension -- going beyond protecting the surrounding environment to safeguarding brands and employees, and providing customers with safer and sustainable products.
The development of safer products requires product compliance at each stage of the product lifecycle. Regulations such as REACH, GHS and RoHS as well as customer demand are redefining what product compliance means and making it an increasingly important factor in everything from materials procurement to completed product distribution. Today, noncompliance poses substantial financial and safety risks, including inability to sell in global markets, unmet customer demand, delayed shipments and revenue loss throughout the entire supply chain.
RETHINKING IS REQUIRED
Many companies begin adhering to legislation during market rollout, after a product is fully developed, only to learn the product isn't in compliance. This tactical and reactive approach limits an organization's options and can be costly to remedy.
The strategic approach (Figure 1) is designing for sustainable products. This means checking for compliance early and often. Regulatory data are a necessary component in all operations including product lifecycle management, supply chain management, order management, sales and operations, as well as government, risk and compliance activities. Product compliance must be integrated into business processes, best practices and information management systems.
A company planning to expand into new markets must make product compliance a strategic initiative in its overall plan. For example, Vlissingen, Netherlands-based Thermphos International realized that lack of compliance would limit its growth. One of the largest producers of phosphorus, phosphoric acid, phosphates, phosphonates and phosphorus derivatives, it supplies worldwide customers that serve a wide variety of markets. "Thermphos was looking to integrate product compliance across all of its business processes so that we can be ready for new legislation as well as geographic expansion," notes Rene de Kok, its REACH coordinator.
THE CHALLENGES
Product compliance has become very complex and costly. Regulations and standards are evolving rapidly and globally. So, a company must promote product stewardship and information technology (IT) that look forward, anticipate change and have a flexible vision.
Even when products are designed to be compliant, extensive documentation and product traceability throughout the entire supply chain remain essential. A company must consolidate information from multiple sources, generate new information and share information with the supply chain. This presents complex IT and business process challenges to produce the necessary and accurate documentation as well as automate communication throughout the supply chain.
"As a part of our continued commitment to environmental improvement and providing sustainable cleaning solutions, we knew that we needed to integrate product compliance into our product lifecycle and supply chain processes," says Gary Arrand, IT director, DuBois Chemical, Sharonville, Ohio.
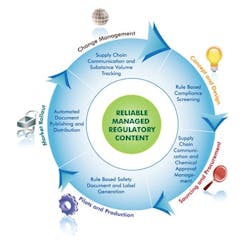
When compliance is considered from the beginning, executives have better insights into risks such as revenue impact, product exemptions, supply chain exposure, inventory vulnerability and the useful life of a particular product. To mitigate corporate risk and have the maximum number of options at minimal cost, an effective product stewardship program begins with product compliance at each stage of the product lifecycle (Figure 2). So, let's now look at these stages.
CONCEPT AND DESIGN
Incorporating product compliance early in the product lifecycle will help a company design more-sustainable and eco-friendly products without increased cost or time to market by ensuring it designs in approved chemical substances from the start. Providing cross-organizational access to a vast and current regulatory-knowledge base and toxicological data standardizes the way product design teams gather and analyze compliance information across business divisions, product lines and geographically distributed teams, enabling more organizational agility during the concept and design process.
At this stage, a company must identify any global regulatory constraints. It must assess raw materials for formulations and identify appropriate labeling requirements. Rules-based compliance screening helps avoid costly last-minute reformulation or repackaging of products by understanding the regulatory business risks associated with material composition based on the intended use. The firm also must create a detailed plan for disposal and end-of-product use.
One of the biggest challenges facing a company at this stage is that product stewardship regulations and standards are changing at an unprecedented rate.
Tips to help keep up with the accelerated pace of change:
• Ensure all data collected and documents developed are in a standard, auditable format. That way any information captured can be re-used in other formats as requirements evolve.
• Develop IT systems that are standardized, flexible and on-demand so that investments are preserved.
• Resist a "not invented here" and "we are unique" attitude. Be open to outsourcing to a specialist in regulatory compliance.
SOURCING AND PROCUREMENT
Once a product has been conceptualized and designed, a company must be able to source sustainable and compliant raw materials from suppliers. For each raw material, important information on composition must be identified to support regulatory requirements.
More specifically, REACH legislation requires manufacturers and importers to have a thorough knowledge of every substance used in the manufacture of an article or preparation. This means the manufacturer must know whether its upstream suppliers have registered or intend to register any substance subject to REACH used in quantities exceeding 1 ton/yr.
A company must enable collaboration between procurement and regulatory teams by implementing compliance-based purchasing workflow as well as continually monitor, assess and manage supplier risk. By leveraging a central collaboration portal, a procurement organization can gain better visibility and control over supplier and raw material data, and reduce operational costs by automating the process of capturing supplier information.
A supplier should provide a "Full Material Declaration" during the procurement process. However, the reality is that many are reluctant to disclose the information for a variety of reasons -- it's not readily available, volumes are low, or they lack the financial or people resources to produce the information until a key customer demands it.
Tips to help gain full material declaration:
• Educate the supplier on why disclosure is necessary. For a number of banned or approved chemical lists, there's no minimum threshold for exemption of an ingredient. Also, a product often is built from blending multiple raw materials; the percentage of the same ingredient must be added if it appears in multiple raw materials.
• The product stewardship department must work together with the purchasing department to make the disclosure a condition of purchase.
• Commit to a nondisclosure agreement with the supplier.
• Allow a supplier to indicate certain ingredients are proprietary or trade secret and then to withhold specific identification, provided it discloses the hazards and regulatory status.
PILOTING AND PRODUCTION
During the pilot and initial production, a company must develop extensive labeling and safety documentation before market rollout. It's important that all hazardous and controlled materials are identified for proper labeling. Using centralized regulatory content, each set of individual regulatory documentation must be harmonized across all geographic locations to preserve product branding and corporate identity standards. To ensure the safety of workers, the firm must quickly and efficiently write health and safety documents in local languages.
Tips to ensure accurate local labeling:
• Consider local language idiosyncrasies so that critical health and safety warnings aren't lost in translation.
• Take a standardized, centralized and global approach to labeling.
MARKET ROLLOUT
Any launch requires supporting product documentation. With heightened demand for public safety and public awareness of potential exposure and consequences, product documentation has become increasingly complete -- ratcheting up the pressure on a company to provide accurate, detailed information about its products and their potential consequences. Many types of documents must be produced with country-specific requirements. A firm with global distribution channels must ensure regulations are met around the world prior to shipping.
"With operations that span Europe, South and North America, and Asia-Pacific, staying on top of changing regulations that differ between each of these regions was becoming quite a challenge to our team in terms of capacity," notes Dr. Sylvia Huber, head of material safety within the material compliance management department at Klüber, Munich, Germany. "For instance, in addition to the new regulations recently introduced in Europe, Asia-Pacific is currently issuing its own regulations and, in the future, will no longer accept safety sheets and labeling based on European regulations. This region now requires all companies to comply with [its] local version of GHS, and a similar request is expected from South America."
Tip for document accessibility:
• Enable customers, employees, authorities and transportation personnel to view and print safety documents anytime from any geographic location via a secure web site.
CHANGE MANAGEMENT
Most companies today are part of an extensive global supply chain spanning many industries and regions. The reality of such an interdependent environment is that a company's compliance status is greatly impacted by other members in its supply chain. Once a product is introduced, the ability to track products through all stages of the supply chain becomes paramount. The key to an effective traceability system is good communication and management between the successive links in the supply.
REACH legislation requires a manufacturer to understand the uses for its products so appropriate exposure scenarios can be developed. This makes communication with downstream users essential for grasping how and under what conditions products are being used. Over the next eight years as REACH becomes fully implemented, iterative communications will be needed both upstream and downstream.
Tips for automating supply chain communications:
• The built-in interrelations between products, raw materials, substances, and uses dictate the sequence of communication within the supply chain. An automated supply-chain communication system should be able to determine what must be communicated and confirmed with both customers and suppliers.
• Communications should be done electronically and tracked so that follow-up activities meet business and regulatory deadlines.
• Dashboard views and advanced reporting capabilities enable a firm to see the impact of raw material changes, the addition of a substance on the SVHC (substance of very high concern) list or the complete replacement or substitution of a raw material.
AUTOMATING PRODUCT COMPLIANCE
Implementing compliance into the product lifecycle is an ongoing process that poses numerous challenges. At the core of any product compliance solution is the regulatory content. Without accurate data, a company can't fully achieve product stewardship goals. The firm must incorporate and automate the same, centralized data throughout its business processes to ensure consistent and efficient decision-making.
In this new era of sustainability, the entire value chain is responsible for the compliance of substances and finished products. A company must make product compliance a strategic initiative -- to design in approved substances at the beginning of the product lifecycle, plan for a product retirement, and work with compliant supply chain partners. Sophisticated communication and traceability tools are essential to engage with these partners as part of the extended compliance team.
Only when a company fully automates product compliance throughout the product lifecycle and value chain can it achieve optimal product stewardship performance.
FRANK ARCADI is St. Laurent, Que.-based senior director, EHS and sustainability solutions, for IHS, Englewood, Colo. E-mail him at [email protected].