Evaluate reactivity with limited resources
Level 1 prescreening
Level 1 prescreening assembles information needed for a hazard assessment and anticipates potential hazards. Material Safety Data Sheets (MSDSs) are always available but often are incomplete and should be supplemented with other sources to obtain reliable information, such as physical and thermodynamic properties, reactivity, flammability and toxicity, on each chemical. Also important is compatibility information, which is available from a variety of sources; for instance, the U.S. National Oceanic and Atmospheric Administration offers a Chemical Reactivity Worksheet (CRW) program for each chemical pair of more than 6,000 chemicals. (http://response.restoration.noaa.gov/chemaids/react.html). Many reference books and literature articles provide free of charge MSDSs as well as other Level 1 information. Brethericks Handbook of Reactive Chemical Hazards[3], for example, is available in libraries and includes numerous records of incidents that are valuable for anticipating potential reactivity hazards.
Chemical bonds and structures. As part of prescreening, a look at the molecular structure, the fundamental basis for intrinsic properties, is useful for anticipating potential hazards. Some chemical bonds and structures, such as unsaturated bonds (acetylene, butadiene) that are not in benzene rings, are more likely than others to exhibit high reactivity or instability. Other examples include certain compounds containing nitrogen-nitrogen bonds (azo compounds, azides) or oxygen-oxygen bonds (peroxides, peroxyacids) and certain structures containing metals (metal fulminates, arylmetals) or halogens (halamides, halogen azides). Compounds with strained rings, such as ethylene oxide, are less stable and have a greater tendency to react to a more stable state with a release of energy. Extensive examples of bonds and structures are provided in Brethericks [3], the Center for Chemical Process Safety (CCPS) Guidelines [4] and Lees Loss Prevention [5].
Chemical reaction types. It also is important to gather information on the types of expected reactions and their behavior, which include oxidation, oxidation-reduction, polymerization, combustion, decomposition, rearrangement and autocatalytic [4, 6].
Physical processes. Heat generation or consumption by such processes can significantly affect system temperature and, therefore, chemical reaction rate. Examples of such physical processes that are part of Level 1 information are melting, vaporization, wetting, adsorption, hydration, mixing, agitation, combining, grinding and distillation.
After Level 1 information is gathered, experimental and computational screening tests should be performed to identify and characterize basic hazards, including self-reactivity or thermal stability, oxidizers, peroxide formation sensitivity, spontaneous combustibility, reactivity with air, shock sensitivity and incompatibility [7]. A useful guide for identifying and managing basic hazards [8] is available free from the OSHA website on reactive chemical hazards (http://www.osha.gov/SLTC/reactivechemicals/). Even though the necessary tests incur more costs than Level 1 information searches, they still only require limited resources as part of hazard assessment to design and implement reliable chemical processes.Included in a hazard assessment are scenarios or chains of events, developed to determine how the identified hazards can threaten process equipment and personnel. For this analysis, sensitivities of the chemicals to likely contaminants and conditions, as measured in screening tests, together with lessons learned from investigations of previous incidents involving the chemicals are extremely valuable. For each developed scenario, the potential consequence severity and the likelihood of each event are analyzed as needed to manage risk.Experimental screening. Many calorimetric devices are available for testing reaction behavior at process and likely upset conditions. A Differential Scanning Calorimeter (DSC) is useful for examining a few milligrams of sample over wide ranges of temperature to identify exothermic processes and estimate evolved heat [4]. Other small calorimeters, such as the ARSST [9], test a few grams of sample to estimate reaction behavior, including heat of reaction, and pressure change. Computational screening. Although accurate measurements of reaction energies or heat are expensive, reaction energy can be calculated by subtracting the heats of formation of the products from those of the reactants. Experimental heats of formation are available for many molecules from the National Institute of Science and Technology (NIST) Webbook (http://webbook.nist.gov). Reaction energy can be used to develop parameters for reactivity prediction methods. An example reactivity parameter is the Computed Adiabatic Reaction Temperature (CART), which is the maximum temperature under adiabatic conditions from the heat of reaction and heat capacity of reaction components [10].Especially for molecules with unusual functional groups and reactive intermediates, a calculation method is a convenient way to determine heats of formation when experimental values are not available. High-level quantum chemical calculations can rival experimental values in accuracy but can be time consuming and expensive and often are not required. As screening tools, however, empirical and semi-empirical desktop software methods with usually acceptable accuracy are available at low cost. The CHETAH computer program, which employs the Benson group additive method, is useful for estimating gas-phase heats of formation [11]. MOPAC within the ChemOffice Ultra 2004 programs (http://www.cambridgesoft.com/) also calculates gas-phase heats of formation. MOPAC includes semi-empirical quantum methods, in which many of the electron interaction terms are neglected or replaced by empirical parameters based on measured data. These widely applicable methods are inexpensive and require only about a minute on a personal computer to calculate a heat of formation [7].Employing a multi-leveled approach to hazard assessment, information gathering and hazard identification is the critical preliminary to hazard characterization. Each step along this assessment route should lower the overall risk. Examples of basic reactive chemical hazards from the CSB 167 incidents [1], summarized below, demonstrate that relatively low-cost Level 1 and 2 methods could have identified, and semi-quantitatively characterized, basic hazards that were involved in each incident. CSB Incident No. 4, spontaneous combustibility. A fire appeared in a scrubber tank that contained mineral oil and alkyl aluminums, which are pyrophoric and ignite on contact with air or water. Although it could have been determined from a Level 1 search, the propensity of water-contaminated alkyl aluminums to self ignite and be an ignition source for mineral oil was not identified, so personnel had not prepared procedures for safe handling and use of these chemicals. CSB Incident No. 167, oxidizer. Personnel were unclogging a dust collector that removed combustible dust, including ammonium perchlorate, a powerful oxidizing agent. The personnel were not aware of the dust-explosion initiation potential of ammonium perchlorate, which could have been discovered from a Level 1 search.CSB Incident No. 11, thermal runaway polymerization, shock sensitivity. An overheated tank containing 80,000 gallons of acrylic acid required extensive spraying with water to prevent explosion. Acrylic acid is chemically stable under normal storage and handling conditions, but is sensitive to peroxide formation and exothermic polymerization in the presence of heat or contaminants that can deactivate peroxide formation inhibitors. Organic peroxides sensitivity to heat, shock and friction has resulted in many unexpected explosions. Also, with its relatively high freezing point of 14°C, acrylic acid often partly solidifies, with the peroxide formation inhibitor remaining in the liquid phase. Uninhibited acrylic acid polymerizes exothermically at ambient temperature and may accelerate to an explosive state. This basic information about acrylic acid, organic peroxides and potential runaway behavior could have been discovered from a Level 1 search that included records of previous incidents. Level 2 screening or higher-level tests could characterize the potential hazards and further reduce risk.CSB Incident No. 16, reactivity with air, self reactivity, incompatibility. NaK used as thermometric fluid spilled into a furnace. Personnel were not aware that NaK reacts with air to form KO
Releases of flammable gases that disperse and encounter ignition sources [13];
Aerosols produced from leakage of industrial fluids to ambient pressure from higher pressures [14]; and
Dust accumulations that can form airborne dust clouds that disperse and explode [15].
Level 3 detailed reactivity tests
Screening tests satisfy or at least greatly simplify minimum requirements for a hazard assessment. In some cases based on Level 1 and Level 2 information, however, additional quantitative information using more detailed computations or experimental tests may be required to reduce risk to an acceptable level. Computational tests include quantum methods such as HF, G2 and B3LYP [16], while experimental methods include tests under adiabatic conditions using adiabatic calorimeters such as the APTAC or ARC [4]. These tests require increased resources, but they can explore sensitivities of the chemicals and provide more accurate values of reaction heats and activation energies for estimating hazard evaluation parameters, such as time to maximum reaction rate, which are useful for risk quantification and reduction. The following example from the CSB 167 incidents illustrates the value of Levels 2 and 3 testing.
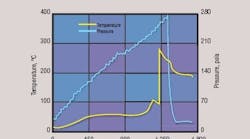
CSB Incident No. 1, self reactivity, thermal decomposition. Nylon polymer in a process vessel had decomposed to carbon dioxide and other gases. Because the foamy polymer fluid had blocked vessel tubing, operators opening the vessel were not aware of the trapped pressure. The partially unbolted cover blew off and the released hot fluid ignited. Following the incident, adiabatic calorimetric testing showed that the thermal decomposition of the polymer was endothermic with significant pressure generation (Figure 1) [17]. Closed-cell testing at Levels 2 and 3 over a temperature range that included the primary and secondary decomposition reaction systems could have identified and measured pressure formation behavior, including maximum pressure and pressure generation rate. Other reactivity characterization information for hazard assessment is onset temperature for significant decomposition, the difference between the main process temperature and the decomposition onset temperature and, from Level 3, the time to the maximum pressure generation rate under adiabatic conditions.
Thermal decomposition of a nylon polymer generated significant pressure that ultimately led to a fluid release and fire.
Tools you can apply
Before hazards can be controlled, they must be identified. The multi-tiered approach and methods we have outlined can play a key role in identifying reactivity risks, without requiring extensive resources. The cited CSB incidents underscore the value of the approach. Basic reactivity hazards should have been uncovered from a Level 1 information search in all but the last incident case study. If Level 1 information had been employed, followed by Level 2 screening for hazard identification and initial characterization, the number and severity of incidents should have been greatly reduced. Level 3 tests for more detailed characterization require greater resources, but just the knowledge that additional information beyond Level 2 is needed helps to determine whether a process can be performed economically at a manageable level of risk or must be modified or abandoned. Additional information is available from many sources including the periodically updated websites of OSHA (www.osha.gov/SLTC/reactivechemicals/), and the Mary Kay OConnor Process Safety Center (http://process-safety.tamu.edu).
| Sam Mannan and William Rogers co-teach a course concerning reactive chemical hazards as part of a continuing education program provided by the Mary Kay OConnor Process Safety Center, Texas A&M University, College Station, Texas. For more information concerning available courses, call (979) 458-1863 or visit http://process-safety.tamu.edu. |
W. J. Rogers is a research scientist at the Mary Kay OConnor Process Safety Center at Texas A&M University, College Station, Texas. E-mail him at [email protected].
C. Wei was a research assistant at the Mary Kay OConnor Process Safety Center, and is currently employed by DNV Consulting. E-mail her at [email protected].
M. S. Mannan is director of the Mary Kay OConnor Process Safety Center. E-mail him at [email protected].