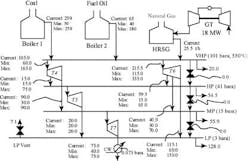
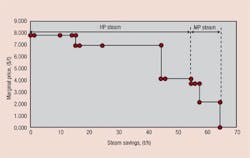
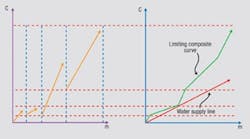
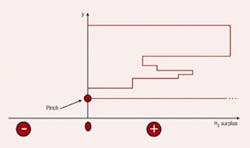
Any investigation of potential improvements in energy integration should start with a site’;s utility system. This is the only way to establish the true incentives to enhance the energy performance of individual processes. Figure 1 illustrates a typical site setup in which different production units are linked to a common utility system that distributes steam at various pressures. The process units use this steam and, in some cases, generate steam from waste heat and feed it to steam headers. Backpressure steam turbines might generate power from the expansion of steam from high to low pressures. (The steam turbines in Figure 1 also feature some condensing power generation.) Large sites might also employ gas turbines. Power might be imported from centralized power generation or, in some cases, exported from the plant. On such a site, what is the true value of steam saving? The answer turns out to be not so obvious.Figure 1. Typical site steam system
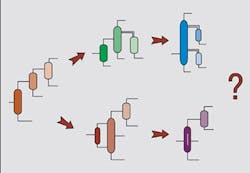
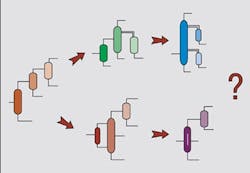
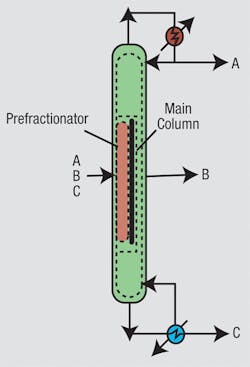
Distillation system design also is attracting increasing attention. Before we get into this, it is useful to distinguish between simple and complex columns. A simple column has a reboiler and a condenser, and splits a single feed into two products. Although different configurations are possible when using simple columns, the design problem still is a manageable size. However, separations often require more complex arrangements, including thermal coupling, side strippers, side rectifiers, prefractionators and dividing-wall columns (Figure 5). As the number of products increases, the number of possible complex column configurations explodes. In addition to this problem, we need to choose the most appropriate pressure for each separation in a complex arrangement and to consider heat integration simultaneously.Figure 5. Complex distillation
Tackling reactions and beyond
Obstacles certainly need to be overcome in established areas of process integration — and new areas pose enormous challenges. Little work has taken place in new areas to develop methods that can be exploited commercially.
For instance, process integration of reactions systems is still in its infancy. Reactor design generally considers the reactor to be in isolation even though, in most cases, the design interacts closely with the rest of the process. Taking this a step further, catalyst design often occurs earlier and without too much consideration of reactor design. Yet, the design of the catalyst, reactor and separation system should be carried out simultaneously and in an integrated fashion. This is particularly important when the separation system is extremely complex and costly, as is the case with many azeotropic systems. Biochemical reaction systems present similar and, in many ways, more daunting challenges due to the difficulties in modeling such systems at a level of sophistication appropriate for process integration methods.
The process integration of separation systems other than distillation has hardly been considered. Membranes, crystallization and solids-processing operations in general all require further development.
In addition, more attention needs to be paid to batch processes. Whereas heat integration of batch processes usually is not worthwhile, there still is significant potential for improvement through better process integration. The time element in batch processes provides flexibility in design and operation. However, this flexibility is often taken as an excuse for not doing a thorough and optimized design. In these applications, data are often the biggest problem. How can we design and optimize such a process if we don’;t have basic information such as reaction kinetic data? Such problems require experimental investigation (and the design of the experiments) to be conducted in parallel with the design of the process and its integration. The experimental design and the process design must be integrated in a coordinated activity.
The trend in process integration has been moving away from using graphical systems and moving toward employing automated optimization techniques. Graphical techniques still have a role to play, but are limited. We need the power of mathematics combined with graphical displays for better understanding. Lack of software for the newer areas of process integration is a bottleneck. There is plenty of commercial software for energy integration. More recently, software has come on the market for water- and hydrogen-system integration and the design of distillation schemes.
However, software on its own is not enough. To take the fullest advantage of process integration, engineers need to be educated about newer techniques. Although university curricula are already crowded, this education must start at school and continue through professional training. Otherwise, process engineers will miss significant opportunities to improve designs.
Dr. Robin Smith is a professor in the School of Chemical Engineering and Analytical Science at the University of Manchester. A Chartered Engineer, Fellow of the Institution of Chemical Engineers and Fellow of the Royal Academy of Engineering, he is the author of the upcoming book, “Chemical Process Design and Integration.” E-mail him at [email protected] or [email protected].
References
1. Hohman, E.C., “Optimum Networks of Heat Exchange,” Ph.D. Thesis, University of Southern California, Los Angeles (1971).
2. Huang, F. and R.V. Elshout, “Optimizing the Heat Recovery of Crude Units,” Chem. Eng. Progr., Vol. 72, p. 68 (Month needed 1976).
3. Linnhoff, B., D.R. Mason and I. Wardle, “Understanding Heat Exchanger Networks,” Comp. Chem. Eng., Vol. 3, p. 295 (1979).
4. Umeda, T., J. Itoh and K. Shiroko, “Heat Exchange System Synthesis,” Chem. Eng. Progr., Vol. 74, p. 70 (Month Needed 1978).
5. Umeda, T., T. Harada and K. Shiroko, “A Thermodynamic Approach to the Synthesis of Heat Integration Systems in Chemical Processes,” Comp. Chem. Eng., Vol. 3, p. 273 (1979).
6. Umeda, T., K. Niida and K. Shiroko, “A Thermodynamic Approach to Heat Integration in Distillation Systems,” A.I.Ch.E.J., Vol. 25, p. 423 (1979).
7. Itoh, J., K. Shiroko and T. Umeda, “Extensive Application of the T-Q Diagram to Heat Integrated System Synthesis,” presented at Intl. Conf. on Proc. Systems Eng., Kyoto, Japan (1982).
8. Linnhoff, B. and E. Hindmarsh, “The Pinch Design Method of Heat Exchanger Networks,” Chem. Eng. Sci., Vol. 38, p. 745 (1983).
9. Townsend, D.W. and B. Linnhoff, “Heat and Power Networks in Process Design,” A.I.Ch.E.J., Vol. 29, p. 742 (1983).
10. Klemes, J., V.R. Dhole, K. Raissi, S.J. Perry and L. Puigjaner, “Targeting and Design Methodology for Reduction of Fuel, Power and CO2 on Total Sites,” J. Appl. Therm. Eng., Vol. 17, p. 993 (1997).
11. Biegler, L.T., I.E. Grossmann and A.W. Westerberg, “Systematic Methods of Chemical Process Design,” Prentice Hall, Englewood Cliffs, N.J. (1997).
12. Varbanov, P.S., S. Doyle and R. Smith, “Modelling and Optimization of Utility Systems,” Trans. Inst. Chem. E., Vol. 82A, p. 561 (2004).
13. Varbanov, P.S., S. Perry, Y. Makwana, X.X. Zhu and R. Smith, “Top-level Analysis of Site Utility Systems,” Trans. Inst. Chem. E., Vol. 82A, p. 784 (2004).
14. Asante, N.D.K. and X.X. Zhu, “An Automated and Interactive Approach for Heat Exchanger Network Retrofit,” Trans. Inst. Chem. E., Vol. 75, p. 349 (1997).
15. Zhu, X.X. and N.D.K. Asante, “Diagnosis and Optimization Approach for Heat Exchanger Network Retrofit,” A.I.Ch.E.J., Vol. 45, p. 1,488 (1999).
16. El-Halwagi, M.M. and V. Manousiouthakis, “Synthesis of Mass Exchange Networks,” A.I.Ch.E.J., Vol. 8, p. 1,233 (1989).
17. Takama, N., T. Kuriyama, K. Shiroko and T. Umeda, “Optimal Water Allocation in a Petroleum Refinery,” Comp. Chem. Eng., Vol. 4, p. 251 (1980).
18. Wang, Y.P. and R. Smith, “Wastewater Minimization,” Chem. Eng. Sci., Vol. 49, p. 981 (1994).
19. Doyle, S.J. and R. Smith, “Targeting Water Reuse with Multiple Contaminants,” Trans. Inst. Chem. E., Vol. B75, p. 181 (1997).
20. Alves, J.J. and G.P. Towler, “Analysis of Refinery Hydrogen Distribution Systems,” Ind. Eng. Chem. Res., Vol. 41, p. 5,759 (2002).
21. Gadalla, M., M. Jobson and R. Smith, “Increase Capacity and Decrease Energy in Existing Refinery Distillation Columns,” Chem. Eng. Progr., Vol. 99, p. 44 (Apr. 2003).
22. Kaibel, G., “Distillation Columns With Vertical Partitions,” Chem. Eng. Technol., Vol. 10, p. 92 (1987).
23. Triantafyllou, C. and R. Smith, “The Design and Optimization of Fully Thermally-coupled Distillation Columns,” Trans. Inst. Chem. E., Vol. 70A, p. 118 (1992).
24. Becker, H., S. Godorr, H. Kreis and J. Vaughan, “Partitioned Distillation Columns – Why, When and How,” Chem. Eng., Vol. 108, p. 68 (Jan. 2001).
25. Shultz, M.A., D.G. Stewart, J.M. Harris, S.P. Rosenblum, M.S. Shakur and D.E. O’;Brien, “Reduce Costs with Dividing-Wall Columns,” Chem. Eng. Progr., Vol. 98, p. 64 (May 2002).
26. Doherty, M.F. and M.F. Malone, “Conceptual Design of Distillation Systems,” McGraw-Hill, New York (2001).