Like titanium, zirconium is a somewhat esoteric metal. Although it has been used in chemical processing applications for more than 40 years, the metal is seeing increased use in highly corrosive applications because of its resistance to most mineral acids, strong alkalis, organic acids and salt solutions.
Corrosion costs the United States $276 billion per year in direct costs, some 3 percent of GNP, $550 billion per year in indirect costs, and presents long-term environmental risks.
Zirconium can be fabricated, machined and welded using conventional equipment, and is used in heat exchangers, condensers, stripper columns, reactor vessels, column internals, pumps, valves and piping systems.
This article summarizes the metal's key performance characteristics in various acid systems, and shows how it is being applied in the chemical processing industry to reduce equipment maintenance requirements and downtime, prevent product contamination and increase process efficiency.
Zirconium offers excellent resistance to many strong acids, including nitric, hydrochloric and 70 percent concentrated sulfuric acid, as well as most organic acids. It also resists most chloride salt solutions and will stand up to alkaline environments.
However, the metal is vulnerable to attack by hydrofluoric acid, acidic oxidizing chloride solutions such as ferric or cupric chloride solutions, red fuming nitric acid, concentrated sulfuric acid, aqua regia and wet chlorine environments.
Like titanium, zirconium forms a protective oxide film in many corrosive applications. This film is "self-healing" in the presence of air or water, and protects the metal's surface from chemical and mechanical attack. In addition, zirconium can be thermally treated in air, at 550 Degrees C for 4-6 hours, to increase the thickness and durability of this oxide film, resulting in a surface that will resist erosion and abrasion. This resultant surface oxide layer can be particularly useful for components such as fasteners, column trays, rotating shafts, impellers and other equipment exposed to abrasive or erosive conditions.
The metal shows low toxicity in most environments, with a very low rate of corrosion. Other materials may also show low corrosion rates over the short term, but the long-term impacts can be dramatic. Consider, for example, the exposed metal surface of a typical 500- m2 heat exchanger made of Alloy 316 stainless steel. If this heat exchanger corrodes at the rate of 2 mils/year, it will leach 350 g of iron, 80 g of chromium and 55 g of nickel per day. After even a few years of service at a fairly low corrosion rate, the equipment is releasing a significant amount of metal impurities into the final product.
Alloys that make the grade
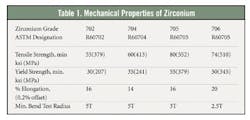
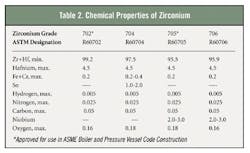
Zirconium alloys used for chemical processing are available in four ASTM grades: Zr702, Zr704, Zr705 and Zr706. Only Zr702 and Zr705 are commonly produced and currently approved for use in ASME boiler and pressure vessels. Tables 1 and 2 show the mechanical and chemical properties of commercial zirconium alloys. Zr702 has the lowest strength and generally the best corrosion resistance of the chemical processing grades. The Zr705 alloy, with 2 percent to 3 percent niobium, is stronger and more ductile.Nitric acid: No match for Zr
Zirconium offers excellent corrosion resistance in nitric acid environments, at acid concentrations exceeding 95 percent and temperatures well above boiling. The metal was first applied in a nitric acid cooler condenser in 1979 at Mississippi Chemical's plant in Yazoo City, Miss.The condenser was constructed using Zr702 tubing and a zirconium explosive clad 304L stainless steel tubesheet. The projected life of the unit was 20 years, with an expected $200,000 per year in savings.
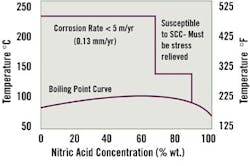
Figure 1 shows an iso-corrosion curve for zirconium in nitric acid. As the curve indicates, the metal shows exceptional resistance, but it is susceptible to cracking at acid concentrations above 70 percent. Proper equipment design and stress-relief heat treatments can mitigate localized attack at higher acid concentrations.
Figure 1. Zirconium in HNO3: Vulnerable to Stress
This iso-corrosion curve shows that Zirconium offers resistance at a wide range of concentrations and temperatures, but is vulnerable to stress corrosion cracking, requiring design features and stress relieving heat treatments.
H2SO4: Watch the welds
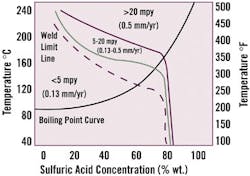
One of zirconium's first commercial chemical processing applications was in sulfuric acid environments. The metal is one of few materials that resists the acid at concentrations up to 70 percent, and above boiling temperatures. Recently, zirconium equipment has found increased use in processes used to make methyl methcrylate and alcohols, in certain organic synthesis reactions, as well as in sulfuric acid recovery systems and pickle tank heaters. Its use in grid coils for sulfuric acid pickle baths has allowed a more efficient, environmentally safer method of heating acid.Despite its strong resistance (see Fig. 3), the metal's welds and heat-affected zones (HAZs) are vulnerable to corrosion at higher acid concentrations. This susceptibility is due to formation of continuous networks of second-phase intermetallic compounds, which precipitate in alpha grain boundaries as the weld cools. Heating the welds at 630-780 Degrees C for 30 minutes to two hours will generally improve corrosion resistance in the weld and HAZ.
Figure 2. Zirconium in H2SO4: Heat Treatment Relieves Weld Worries
Zirconium is vulnerable to attack at high sulfuric acid concentrations, as precipitates form within the weld. Heat treatment will improve resistance.
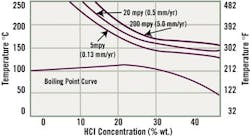
Figure 3. Zirconium in HCI: Vulnerable to High Concentrations
Zirconium is vulnerable at high HCl concentrations. For equipment being developed for HCl applications, care must be taken to ensure that the metal surface does not come in contact with iron or copper during fabrication. A chemical conditioning pre-treatment should be used to be on the safe side.
HCl: Hold the ions
Zirconium is used in numerous processes that involve hydrochloric acid, and the metal's corrosion resistance approaches that of tantalum in pure HCl media. Applications include production of azo dyes and fine chemicals, geothermal applications to acidify salt solutions and in hydrochloric evaporators.You'll note in Fig. 3, however, that the metal is susceptible to localized corrosion attack, including pitting, stress corrosion attack and intergranular attack in oxidizing chloride solutions. Zirconium should never be used where ferric or cupric ions are present.
In addition, care should be taken during the fabrication of zirconium vessels destined for hydrochloric acid service to ensure that no iron or copper is embedded on the metal surface. Consider chemical conditioning pre-treatment prior to placing zirconium equipment into hydrochloric acid service.
Chloride salt resistance
In chloride salt solutions, most materials are susceptible to localized corrosion attack such as pitting, crevice corrosion or stress corrosion cracking. Zirconium, however, has good resistance to severe chloride environments, even at elevated temperatures. In addition to seawater, brackish and pure waters, zirconium is very resistant to a wide range of chloride salt solutions. Zirconium has limited use, however, in ferric or cupric chloride solutions and will undergo localized corrosion, including pitting or intergranular attack and stress corrosion cracking in these solutions. The presence of the ferric or cupric ions will cause the localized breakdown of the metal's passive oxide layer.Excellent with organics
Zirconium offers excellent resistance to organic media including formic acid, acetic acid, hydroxyacetic acid, lactic acid and urea. Process vessels for the production of acetic acid have been the single largest organics application for zirconium. Only a few conditions will cause zirconium to corrode in acetic acid. These include the presence of cupric ions, free chlorine and extremely low water content. Under highly stressed conditions, a minimum of 650 ppm water is needed to prevent stress corrosion cracking in acetic acid.Zirconium also is highly resistant to attack by caustics, including sodium and potassium hydroxide, allowing design of equipment that simultaneously resists acidic and alkaline environments. In summary, zirconium can be a cost-effective, highly corrosion-resistant material of construction for chemical processing applications. It has been successfully applied in many severely corrosive environments. In many cases, the metal has not only helped reduce downtime, but lowered maintenance costs, increased process efficiency and eliminated corrosion products in the final product.
Richard Sutherlin is manager of technical services at Wah Chang. A Metallurgical Engineer and a registered Professional Engineer in the state of Oregon, he has more than 25 years experience in the field of corrosion, welding and fabrication of reactive and refractory metals.
|
References 1. Corrosion Costs and Prevention Strategies in the United States," Pub. No. FHWA-RD-01-156, 2002. 2. Webster, R.T., "Zirconium Cost Effective Uses in Corrosive Applications," Paper 459, Corrosion/87, San Francisco, Ca., 1987, pp.4. 3. Yau, T.L., Webster, R.T., Zirconium in Sulfuric Acid Applications," Materials Performance, Vol. 25 (2), 1986: pp. 15-17. 4. Frechem, B.S.,Fitzgerald, B.J., Webber, R.G.,et al., "Factors to Consider for using Zirconium in Sulfuric Acid Services," Corrosion/ 95, 1995, pp 6. 5. Yau, T.L., Maguire, M., "Electrochemical Protection of Zirconium in Oxidizing Hydrochloric Acid Solutions," Corrosion, Vol.40, No. 6, pp 289. 6. Yau., T.L., "Zirconium for Seawater and Chloride Solutions," Presented at Fourth Asian-Pacifica Corrosion Control Conference, May 26-31, 1985, Tokyo, Japan 7. Yau, T.L., "Performance of Zirconium and Zirconium Alloys in Organics," Journal of Testing and Evaluation, March, 1996. |