MOF Promises Cheaper Ethylene Separation
A modified metal-organic framework (MOF) can efficiently separate purified ethylene from a mixture with ethane, reports a team of researchers from the National Institute of Standards and technology (NIST) and The University of Texas at San Antonio (UTSA).
“Most MOFs that have been studied grab onto ethylene rather than ethane. A few of them have even demonstrated excellent separation performance, by selectively adsorbing the ethylene. But from an industrial perspective you would prefer to do the opposite if feasible. You want to adsorb the ethane byproduct and let the ethylene pass through,” notes Wei Zhou of the NIST’s Center for Neutron Research.
“Finding ways to break the strong bond that forms between carbon and hydrogen …allows you to create a lot of valuable new materials,” adds UTSA professor Banglin Chen, who led the team. “We found previous research that showed that compounds containing iron peroxide could break that bond.”
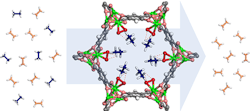
So, the researchers modified the walls of a well-studied MOF (MOF-74) to contain a compound structure that would attract ethane from the mixture. The iron-based MOF is decorated with peroxo groups that can capture ethane while allowing ethylene to pass through, potentially providing a more efficient and cost-effective way to purify ethylene. Results showed that the first adsorption cycle straightforwardly produces polymer-grade ethylene (99.99% pure) from ethane/ethylene mixtures, demonstrating potential industrial separation with a low energy cost under ambient conditions. A recent article in Science contains more detail.
Figure 1. Etheylene and ethane molecules pass through a ring-shaped MOF structure that attracts and filters out the ethane. Source: W. Zhou, NIST.
“We also think that we can try to add other small groups to the surface, maybe to do other things,” says Chen. “It is quite possible that hydroxide (-OH) and oxide (=O) might initiate stronger interactions with alkanes as well.”
While Zhou says the team’s modified MOF does work efficiently, he admits it will require additional development to see action at a refinery. While separation performance and stability were maintained over five regeneration cycles, the material is quite sensitive to air and moisture and, so, would need protection for long-term usage, cautions Chen.
“We proved this route is promising,” Zhou notes, “but we’re not claiming our materials perform so well they can’t be improved. Our future goal is to dramatically increase their selectivity. It’s worth pursuing further.”
The team currently is working to improve dual functionality with suitable pore sizes to match ethane and specific sites that can induce stronger interactions. “Also, the materials need to be very stable and cost efficient,” adds Chen.
The MOF worked well with feed streams containing CH4, H2 and C2H2 impurities. However, the team has no immediate plans to run tests on streams that contain other low-level impurities commonly found in commercial C2H4 streams. “Currently, we are focused on the scientific part,” says Chen. “We will need to collaborate with industrial partners to have such further development carried out before they can be applied to the true applications.”
This specific MOF is not easily synthesized on a large scale, notes Chen. However, the team recently published a paper in Nature Materials on an MOF that uses a sieving mechanism that can easily be scaled up and also much cheaper. “This one is much more feasible for industrial usage,” he says. That article also concluded: “…The ideal molecular exclusion of ethane from ethylene can be readily achieved by integrating the merits of size/shape match and high rigidity into the pore structure, which may greatly facilitate the very challenging membrane-based separation of such essential hydrocarbons. In principle, pore engineering in MOF chemistry is generally applicable to other types of porous material including porous organic polymers, covalent organic frameworks and hydrogen-bonded organic frameworks.”