Borane Catalyst Opens Up Opportunities
A new nonmetallic catalyst promises to enable economical hydrogen-based substitution reactions that now require expensive or hazardous synthesis routes, e.g., making hydrosilanes from chlorosilanes as well as hydrides from halides, believe researchers at Goethe University, Frankfurt, Germany. The borane catalyst splits hydrogen molecules under mild conditions and should suit broad-scale use, they say.
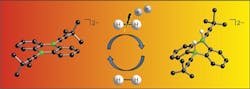
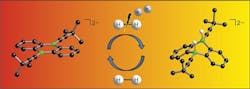
The researchers created the catalyst by introducing boron atoms into a common organic molecule (Figure 1). The catalyst provides ambiphilic metal-like reactivity toward H2; tests so far indicate yields are comparable to conventional metallic catalysts.
Figure 1. Introducing boron atoms into an organic molecule creates an effective catalyst for hydrogen activation. Source: Goethe University.
“What we additionally need is just an electron source. In the laboratory we chose lithium or potassium for this. When put into practice in the field, it should be possible to substitute this with an electrical current,” notes Matthias Wagner, a professor in the Institute of Inorganic and Analytical Chemistry at the university.
[callToAction ]
“We want to further investigate substituent and counterion effects to speed up the H2-activation process and to render the electrocatalyst as stable as possible toward air and moisture. Parallel to that, we will thoroughly investigate its reactivity. One aim is to do hydrogenation reactions (e.g., imine to amine), the other to perform nucleophilic substitution reactions (e.g., element halide to element hydride),” he explains.
Such work already has begun and will last at least two years, he says, with tests using an electrical current instead of lithium or potassium probably starting in the summer of 2017.
The researchers already are working with two major hydrosilane producers on related topics but have not yet discussed this particular system with those companies, notes Wagner. Teaming up with specialists from industry would be the preferred way to handle any eventual pilot-plant trials, he adds.
More details appear in an article in Angewandte Chemie International Edition.