Images Capture Chemical Reactions
At the heart of most industrial chemical manufacturing processes are transformations that occur at interfaces between either the solid/liquid or solid/gaseous phases.
[pullquote]
The analytical techniques available limit detailed understanding of the molecular energetics and conformational dynamics that drive these transformations. So, being able to follow and directly visualize how the structures of molecules change when they undergo complex chemical transformations is a major challenge.
An even bigger challenge is identifying and characterizing reaction intermediates. Not only are these typically highly unstable, but they also have very short lifetimes. Gaining an insight into these intermediates would be a big step in improving the understanding of how important industrial chemical reactions occur.
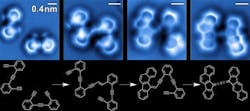
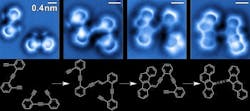
Figure 1. Sequence of images of the steps in the reaction of enediyne molecules on a silver surface. Source: A. Riss/Technische Universität München.
Now, researchers at the Universidad del Pais Vasco/Euskal Herriko Unibertistatea (UPV/EHU) University of Basque Country, Spain, have announced a significant step towards achieving this — they have succeeded both in imaging all the steps in a complex organic reaction and resolving the mechanisms that explain it.
An international collaborative effort, Felix R. Fischer, assistant professor at University of California-Berkeley (UC Berkeley), Calif.; Michael F. Crommie, physics professor, also at UC Berkeley; and Angel Rubio, UPV/EHU professor and leader of the university’s nano-bio spectroscopy research group, led the research.
Together, they imaged and resolved at the single-molecule level the bond configuration of the reactants, intermediates and final products of a complex, organic reaction: a surface-catalyzed cross-coupling and sequential cyclization cascade of 1,2-bis (2-ethynyl phenyl) ethyne on silver.
[javascriptSnippet ]
The team obtained the images of the chemical structures associated with different steps in the reaction cascade using non-contact atomic force microscopy with a particularly sensitive tip: it uses a very fine needle that can detect the smallest bumps on an atomic scale as it absorbs a carbon monoxide molecule that acts like a “finger” on the text to increase its resolution.
“The precise identification of the bond configuration of the intermediate species has made it possible to determine the intricate sequence of chemical transformations along the reaction mechanism from reactants via intermediates to end products and at the same time unravel the microscopic mechanisms behind that intricate dynamic behavior,” notes Rubio.
To analyze their results, the researchers combined advances in numerical calculus and the classical analytical models that describe the kinetics of sequential chemical reactions. This showed it’s not possible to explain the stabilization of intermediates only by considering their potential energy, but that it’s essential to bear in mind the energy dissipation and the changes in molecular entropy, which measures how far a system is organized. The surface, and in particular its interaction with extremely unstable intermediates, play a key role for both the entropy and the dissipation of energy. This, says the researchers, highlights a fundamental difference between surface-supported reactions and gas-phase or solution chemistry.
“Such detailed understanding achieved though the synergy between the imaging of the chemical reactions of a molecule and the latest advances in computer modeling constitutes a fundamental milestone in the analysis of chemical reactions. In fact, many of the limitations in conventional spectroscopic techniques have been surpassed and an unprecedented image has been obtained on an atomic scale of the reaction mechanisms, driving forces and kinetics,” adds Rubio.
He believes this new knowledge may open up countless unexplored fields, including: future designs and optimizations of heterogeneous catalytic systems; development of novel synthetic tools applied to carbon-based nanotechnology; and many biochemical and materials science applications.
Also, in April, Rubio announced that the nano-bio spectroscopy group developed a new route to produce carbyne using double-walled carbon nanotubes to protect the carbon chain. The group’s chain length of more than 6,400 carbon atoms broke the previous record by two orders of magnitude.
Not only did the double-walled carbon nanotubes act as nanoreactors to help the chain grow, but they also added stability — a factor that will be important for future applications of carbyne, says Rubio.
According to theoretical models, carbyne has mechanical properties unmatched by any known material; it even outperforms the mechanical resistance and flexibility properties of graphene and diamond. Furthermore, its electronic properties are pointing towards new nano-electronic applications, such as in the development of new magnetic semiconductors, high power density batteries, or in quantum spin transport electronics (spintronics). However, Rubio points out that to do this it would be necessary to extract these ultra-long, linear carbon chains from the double-walled nanotube containing them and stabilize them in some liquid environment.
Seán Ottewell is Chemical Processing's Editor at Large. You can email him at [email protected].