Better Catalyst for Carbon Dioxide Reduction Beckons
Using a novel approach that integrates quantum mechanics and machine-learning algorithms, researchers from Virginia Tech, Blacksburg, Va., have predicted multimetallic copper alloys with improved efficiency and possibly higher selectivity for electrochemical reduction of carbon dioxide to ethylene.
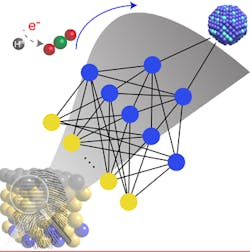
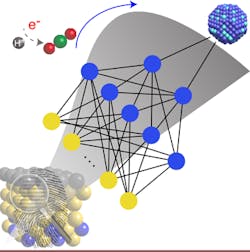
Figure 1. A machine-learning model enables fast and accurate prediction of the surface reactivity of metal alloys that could lead to more efficient catalysts. Source: ACS Publications.
The conventional method of testing mixed blends of metals to find ones most-promising as catalysts is time-consuming and costly, note the researchers. In contrast, their machine-learning chemisorption model enables fast and accurate predictions of the surface reactivity of metal alloys, they say. It captures complex, nonlinear interactions of molecules on metal surfaces through artificial neural networks, thus allowing high-throughput catalyst screening, speeding prediction of novel materials for efficient chemical conversions, explain the researchers. More details on the approach appear in a recent issue of the Journal of Physical Chemistry Letters.
“This is the first example of learning from data in catalysis. We anticipate that this new approach will have a huge impact in future materials design,” says Hongliang Xin, who led the research along with Virginia Tech chemical engineering faculty member Luke Achenie. “This study opens a new way for designing metal-based catalysts with complexities, for example, geometry and composition, promoters and poisons, defects and nano-effects,” he adds.
The researchers now are developing an integrated approach that ties together machine learning with materials properties databases for catalyst design. “We have started the work by collecting data, setting up interface between tools and databases, etc. We would expect to have a beta release in one year,” says Xin.
The team focused on the electrochemical reduction of carbon dioxide on metal electrodes because of the current high interest in developing renewable routes to produce fuels and chemicals.
Validation of the model prediction is currently underway in collaboration with other groups at Virginia Tech and Oak Ridge National Laboratory, Oak Ridge, Tenn.
In addition, the team is using the approach to investigate catalysts related to other electrochemical conversions.
“The approach we have is very general for multimetallic alloys widely used in electrocatalysis typically operated at low temperatures so the active sites can be preserved. Currently, we are applying this approach to Pt alloys for hydrogen PEM fuel cells which could be used in automobile applications in the future,” Xin adds.
In addition, it may identify cheaper alternatives with comparable performance to noble metal-based catalysts, which could be very important for large-scale application of many renewable energy technologies, notes Xin. “We foresee great potential by ‘learning from data’ in those systems because potentially many earth-abundant metal elements could work if tuned properly by forming multimetallics,” he explains.
Other types of catalysts that might suit the approach include complex materials, such as metal oxides, metal organic frameworks, metal dichacogenides, “for which the traditional wisdom of surface reactivity is not applicable and many properties (i.e., input features) are contributing,” says Xin.
However, key challenges remain.
“The developed approach relies on data for training the model. For many catalytic systems, the particle size effects, support effects are dramatic, namely structure sensitivity. To understand those systems and design improved catalysts, we have to understand the origin of reactivity of nanoparticles, supported on typically metal oxides, nitride, carbides, etc. Currently, we do not have enough knowledge about what could be the effective input features and we do not have enough data to let learning algorithms figure it out by itself. With increasing computational power governed by Moore’s law, we will see some breakthroughs in the next five years,” Xin believes.
Many other learning algorithms, such as support vector regression and kernel ridge regression, could work. However, Xin says they chose neural nets because of easy implementation, and its potential for describing complicated systems.