COFs Do Double Duty
Incorporating molecules of carbon dioxide reduction catalysts into covalent organic frameworks (COFs) creates a molecular system that both absorbs carbon dioxide and selectively reduces it to carbon monoxide, say researchers at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory, Berkeley, Calif.
A COF is a porous, compact three-dimensional crystal with an extensive internal surface area that acts much like a sponge, enabling the system to absorb and store large quantities of targeted molecules.
“To date, such porous materials have mainly been used for carbon capture and separation, but in showing they can also be used for carbon dioxide catalysis, our results open up a huge range of potential applications in catalysis and energy,” says Christopher Chang, a chemist with Berkeley Lab’s Chemical Sciences Division, and co-leader of this study.
Omar Yaghi, a chemist with Berkeley’s Materials Sciences Division who invented COFs, has now developed another technique called “reticular chemistry,” which enabled the researchers to “stitch” or bond the molecular backbone of COFs to a porphyrin catalyst with a cobalt center.
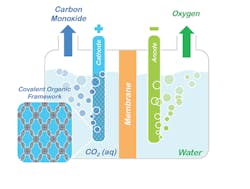
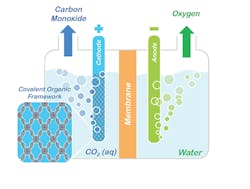
Figure 1. Conceptual model shows how porphyrin COFs could be used to split CO2 into CO and oxygen. Source: Omar Yaghi, UC Berkeley.
“A key feature of COFs is the ability to modify chemically active sites at will with molecular-level control by tuning the building blocks constituting a COF’s framework,” Yaghi explains. “This affords a significant advantage over other solid-state catalysts where tuning the catalytic properties with that level of rational design remains a major challenge. Because the porphyrin COFs are stable in water, they can operate in aqueous electrolyte with high selectivity over competing water reduction reactions, an essential requirement for working with flue gas emissions.”
Results show high catalytic activity — the porphyrin COF has 26 times greater catalytic activity than a molecular cobalt porphyrin catalyst, making it one of the fastest and most efficient catalysts for carbon dioxide reduction, the researchers report. One porphyrin COF can reduce 290,000 molecules of carbon dioxide to carbon monoxide every second. A recent article in Science describes the research in detail.
While the team notes their first-generation catalyst appears to be robust, they also believe there’s room for enhancement. “We are currently further improving the long-term stability of the system in hope that any turnover number may be achievable given the reaction is done for a long enough time. We [are] also interested in improving the catalyst efficiency,” notes Song Lin, a lead author of the Science paper and member of the research team.
Current results indicate clogging or poisoning isn’t an issue, but if such issues arise, tuning the pore size, shape and structure of the active site could address them, the researchers believe.
Work is now focused on making a wider variety of value-added products using COFs and related materials, and improving efficiency in terms of energy input, coupling to solar power, and combining with carbon-capture technologies, says Lin.
In particular, the team is interested in more-reduced carbon products such as methanol as well as carbon-carbon bond-coupled products such as ethylene. “Thanks to the modularity of COFs, different functional building blocks other than cobalt porphyrin may be incorporated to realize this goal,” he notes.
In addition, a combination of cobalt porphyrin with other functional building units may achieve cooperative catalysis that lead to a more energy-efficient process. Lin says this can also be used to construct materials for other energy-related applications.
The hybrid molecular-material platforms likely will suit a broad range of catalytic applications, particularly those that require sustainable solar or electrical input and aqueous compatibility. This includes CO2 reduction to other products than CO, oxygen reduction, water oxidation and proton reduction, “all of which can be used in energy-related processes such as fuel production and fuel cells,” Lin explains.
The team welcomes opportunities to collaborate with industrial firms with regard to practical applications, as well as advice from engineering colleagues on how to integrate the system with industrial plants.