Catalyst Casts New Light on Reactions
Combining visible light with an iridium complex efficiently catalyzes enantioselective C-C bond formation, report researchers at Philipps University Marburg, Marburg, Germany. Their technique replaces the two catalysts (one for photoactivating, and the other for controlling chirality) typically required with a single catalyst — and provides both high yields and high enantioselectivity.
PHOTOREDOX RESEACHERS
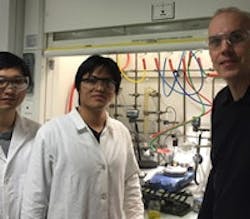
Figure 1. Eric Meggers (right), Haohua Huo (center) and Xiaodong Shen (left) use visible light to make high-purity enantiomers. Source: Eric Meggers.
The researchers reacted 2-acyl imidazole with benzyl bromide to make an α-alkylation product at quantitative yield with an enantiometric excess of 99%. These results were achieved using light from a 14-W energy-saving household lamp, a temperature only slightly higher than ambient, a catalyst loading of 2 mol%, and a reaction time of only 1.5 hr.“This new asymmetric photoredox catalyst, in which the metal center simultaneously serves as the exclusive source of chirality, the catalytically active Lewis acid center, and the photoredox center, offers new opportunities for the ‘green’ synthesis of non-racemic chiral molecules,” they note in a recent article in the journal Nature describing the development. The iridium catalyst is very robust, stable against air and moisture, and suitable for use in a variety of solvents, says Eric Meggers, a professor in the department of chemistry at the university and leader of the research team. “The catalyst can be stored for several months without any problems. The metal-centered chirality is inert and we have not observed isomerizations,” he adds. “We currently are working on applying other classes of electrophiles — for example, to introduce trifluoromethyl and perfluoroalkyl groups since fluorinated compounds are a significant portion of pharmaceuticals,” notes Meggers. “We need to show that this chemistry is applicable to reactions that cannot be performed in other ways.”“I can not tell you too much at this point but we are working on multiple strategies. To put it very simple: You have a chiral photoredox sensitizer that can directly interact with substrates. That’s powerful because it gives you plenty of opportunities to combine redox chemistry with asymmetric catalysis!” While no chemical, pharmaceutical or other firms as yet have contacted the researchers about cooperating on further development of the approach, Meggers is optimistic. “…Since our catalysts are powerful and also easy to make, I am convinced there will be interest in the future.”