New Route Boosts Hydrogen Storage
An electrochemical process to make aluminum hydride (alane) promises to bolster prospects for the "hydrogen economy." The route avoids the cost and byproduct issues that have plagued production of the solid, say its developers — researchers at the Savannah River National Laboratory, Aiken, S.C., of the U.S. Department of Energy (DOE).
Development of the hydrogen economy depends upon efficient and cost-effective hydrogen storage. Storing the fuel as a solid instead of as a liquid or gas promises safety and cost advantages. Several solids meet or exceed the gravimetric or volumetric performance targets set by the DOE for storage but not many provide the thermodynamic and kinetic properties necessary to release hydrogen when needed and then permit economic reloading of the fuel. Alane has the desired properties but regenerating the material has posed technical and economic challenges, note the researchers.
"We believe our research has provided a feasible route to regenerate aluminum hydride, a high-capacity hydrogen storage material," says Ragaiy Zidan, lead researcher on the project. "Our process is cheaper than chemical methods of making alane."
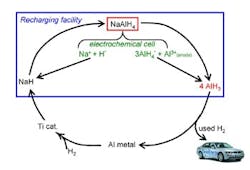
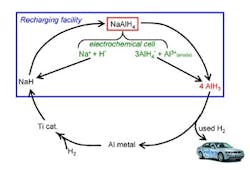
The process uses electrolysis and catalytic hydrogenation of spent aluminum to produce alane at ambient conditions (Figure 1). The electrolysis reaction takes place in a polar solvent such as tetrahydrofuran, while commercial titanium catalyst speeds the hydrogenation. The researchers describe the technique more fully in a recent paper in Chemical Communications.
So far, experiments have been conducted at bench scale (3–4 grams/run) in an inert atmosphere and have involved one regeneration cycle, says Zidan. The catalyst should last around 500 cycles, he believes.
"The biggest challenge is the energy needed to separate alane from the solvent," Zidan explains. "We have immediate plans to change conditions for more-optimum use of energy in regenerating alane. The work is ongoing." The researchers hope that industry will play a role in further developing the technology.
The approach also may suit other applications. "Alane adducts could be used for hydrogen storage or depositing aluminum films on optical and electronic devices, which is useful for industry. The electrochemical method makes it easy to produce these alane adducts," notes Zidan, who adds that the researchers are working on different aspects of adducts.