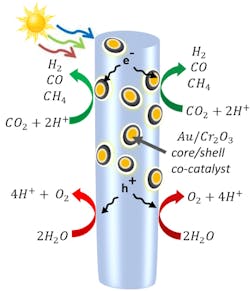
A team of researchers from the University of Michigan (UM), Ann Arbor, Mich., and McGill University, Montreal, have developed a method that generates syngas from carbon dioxide (CO2) and water using only solar energy.
“This opens up many exciting opportunities not previously considered, such as use for feedstocks or as an intermediate for the production of bulk chemicals, fertilizers, pharmaceutical, plastics, solvents and various chemical intermediates (e.g., NH3, methanol, etc.),” says Zetian Mi, professor of electrical and computer engineering at UM, who led the study.
“Our new process is actually pretty simple, but it’s exciting because it’s not toxic, it’s sustainable and it’s very cost effective,” adds Roksana Rashid, who performed the experiments as a doctoral student at McGill.
The solar energy process involves splitting CO2 molecules. For this, the team peppered semiconductor nanowires with nanoparticles made of gold coated with chromium oxide to attract CO2 molecules and bend them, weakening the bonds between the carbon and oxygen. More details appear in an article in the Proceedings of the National Academy of Science.
“What is surprising is the synergy between gold and chromium oxide to make the CO2 reduction to syngas efficient and tunable. That was not possible with a single metal catalyst,” Mi notes.
“Scalability and potential compatibility with standard industrial manufacturing processes is one of the most exciting parts of our work. Our artificial photosynthesis platform utilizes only water, sunlight, and CO2, which is essential for carbon neutrality and sustainability... The semiconductor material we use, gallium nitride and silicon, are the two most-produced semiconductor materials in the world, which makes the manufacturing process of low cost, high throughput, and scalable. And for the catalysts, i.e., particles of Au and Cr2O3, we use very small quantity. In addition, our system is modular, which is well suited for the distribution production of clean chemicals and fuels, which can significantly reduce, or eliminate the transportation costs (which is often higher than the costs of producing chemicals and fuels in some cases),” stresses Mi.
A few challenges remain. Most notably, the catalyst’s susceptibility to poisoning “is a very important topic that needs to study in detail in the future,” he says.
“For future development or large-scale implementation, further studies are needed to improve the efficiency and evaluate long-term stability. Moreover, the effect of CO2 impurity levels, light intensity, reaction temperature and pressure, etc., need to be thoroughly studied, evaluated, and optimized. We do not anticipate any fundamental challenge for the scalability at the materials/systems level,” Mi explains.
The device’s efficiency currently stands at 0.89%; it could be adopted for renewable energy when 10% of the light energy is converted to chemical energy, similar to solar cells, he suggests.
“Artificial photosynthesis technology…is still in a relatively early stage, compared to the mature solar cell panels. While the current efficiency level is relatively low, high efficiency (comparable to the energy conversion efficiency of solar cell panels) can be ultimately achieved.” he believes. Indeed, he foresees the development of artificial photosynthesis devices as efficient as solar cell panels in the next several years. “It may take several additional years for commercialization and market penetration, but the future is bright for this game-changing technology,” he says.
Some of the intellectual property related to this work has been licensed to NX Fuels and NS Nanotech, which were co-founded by Mi.