Researchers from Japan and China have developed a new catalyst that could be used as an inexpensive option to reduce the carbon footprint of the Haber-Bosch ammonia manufacturing process.
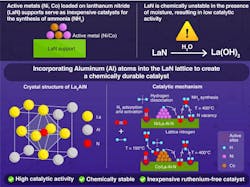
According to a report in a recent Angewandte Chemie, it consists of a metal nitride catalyst containing an active metal — nickel — on a lanthanum nitride (LaN) support that is stable in the presence of moisture.
What the team based at Tokyo Tech, Japan, has done is dope aluminum atoms into the LaN structure and then synthesized a chemically stable La3AlN support containing La-Al bonds. It is these bonds that prevent lanthanum atoms from reacting with moisture.
The new La-Al-N support together with active metals such as nickel and cobalt (Ni, Co) was able to produce NH3 at rates similar to that with conventional metal nitride catalysts and could maintain a stable production when fed with nitrogen gas containing moisture. The Ni- or Co-loaded La-Al-N catalysts showed no distinct degradation following exposure to 3.5% moisture.
Another advantage is the catalyst doesn’t contain ruthenium and as such presents an inexpensive option for reducing the carbon footprint of ammonia production.
One issue that needs to be overcome with the new catalyst, however, is that preparing La-Al-N requires a relatively complicated material synthesis process.
“We first melt La, Al and LaN and then need to anneal the products at 850°C for one week. In addition, the annealing process needs to be progressed in an inert atmosphere or vacuum,” says Yangfan Lu, study co-author and professor with the College of Materials Science and Engineering, National Engineering Research Center for Magnesium Alloys, Chongqing University, Chongqing, China.
One option, trialing alternative rare-earth elements, is not on the table at the moment. “We believe developing a facile synthesis technique is more important than exploring different rare-earth elements,” explains Lu.
“So, while this step is still in the R&D stage it is unlikely to be immediately applied to industry. Instead, our previously studied systems, such as Ru/C12A7:e‒ [a catalytic promoter], which show high catalytic activity for ammonia synthesis, and which can be prepared by an easier method, are considered more suitable for industrial applications at this stage,” he concludes.