Effectively Detect Hazardous Gas Leaks
Gas detector selection is a critical task for fire-and-gas-system engineering design at chemical facilities. Correct detector choice, coupled with proper placement and allocation, underpins fire-and-gas-system effectiveness. A poorly selected gas detector, however reliable and adequate in number and detection coverage, may fail to provide early warning of a hazardous material release.
End users continue to rely on conventional design, using traditional technologies like catalytic, electrochemical and solid-state toxic gas detection. Yet advances in sensor design and the advent of new methods like ultrasonic and open-path detection suggest the need for a reassessment as solid-state combustible, thermal conductivity and calorimetry gas detection have fallen into disuse.
Equally important, the demands placed on gas detection equipment have changed over the years. Much larger process facilities have placed a premium on area monitors, which offer substantial cost savings in installation and maintenance per unit area covered. Similarly, the need to develop safety devices suited for safety integrity level (SIL) 1 and 2 environments has spurred the development of diagnostics, which has favored optical gas detection over passive systems. (Optical gas-detection methods not only provide effective self-checks but also information on sensor degradation that enables predictive health intelligence.)
[callToAction ]
Any updated guide on gas detector selection should consider field experience. Indeed, end users weighing the merits of new methods should take a measured approach: incorporating them only if they offer substantial benefits over legacy devices, and evaluating their service after installation. This article provides a practical list of available gas detectors as well as selection criteria. In developing this, I acknowledge the contributions from the U.K.’s Health and Safety Executive, AIChE’s Center for Chemical Process Safety, and the Council of Gas Detection and Environmental Monitoring.
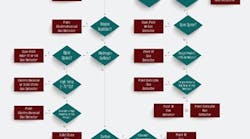
Figure 1. Follow this decision tree to choose the most-appropriate technology for a particular application.
Guidelines
As shown in the selection decision tree (Figure 1), catalytic and infrared (IR) technologies can detect combustible gases. Of the two techniques, only the optical method lends itself well for point and area monitoring. Choose IR devices for detecting a single type of hydrocarbon or simple mixtures. Because they are unaffected by oxygen levels, such detectors are ideal for anaerobic processes, operations with high concentrations of corrosive agents, and areas with constant background of combustible gases. On the other hand, catalytic gas detectors are a better choice for monitoring hydrogen or hydrocarbon mixtures. Because catalytic detectors require oxygen gas concentrations in excess of 10% by volume to operate, they only suit situations where oxygen always is present. Moreover, catalytic gas detectors perform best in applications where the target gas normally is absent.
For monitoring toxic gas leaks in congested spaces, electrochemical and solid-state gas detectors are the instruments of choice. Use electrochemical detectors in applications where the target gas is not normally present and in environments with relative humidity (RH) above 15%. By contrast, solid-state detectors should be used for extreme high temperatures or low humidity applications or where ambient conditions are stable. Oxygen deficiency is best monitored using electrochemical gas detectors sited near the breathing zone. As for open or uncongested spaces, open-path near infrared or ultraviolet detectors offer the widest coverage per device.
Hazardous material releases can take many forms. For pressurized gas releases, choose ultrasonic gas leak detectors to provide the fastest response to a leak. They excel at detection in open, well-ventilated areas where other detection methods may not be wholly effective. For pressurized liquid releases, select air particle monitors.
Gas Detection Options
Plants can turn to a variety of technologies to detect gas leaks. So, let’s briefly look at these options and their pros and cons.
Catalytic and electrocatalytic detection. Traditional detection technologies include point detection systems and area and perimeter monitoring devices. Catalytic detectors often serve as point instruments to measure combustible gases in air at low concentrations. As the combustible gas oxidizes in the presence of a catalyst, the heat of combustion causes the temperature to rise, which increases sensors resistance. A standard Wheatstone bridge circuit transforms the offset voltage caused by the temperature rise into a sensor signal.
While catalytic detectors appear simple in design, they have many advantages. Catalytic detectors are robust, long lasting, economical and dependable; they also compensate for atmospheric factors including humidity, pressure and temperature. In addition, they’re easy to use, install and calibrate, and will function well for years with practically no maintenance. They only require periodic gas calibrations to validate operation. Catalytic detectors are extremely flexible and can measure a wide range of selected gases across a broad array of applications — an advantage unrivaled by other types of detectors.
On the other hand, catalytic gas detectors need oxygen to work, which can be a weakness. The catalytic process requires that hydrocarbon gas be oxidized efficiently — but oxygen levels can impact oxidation efficiency, affecting the accuracy and reliability of the sensor. In addition, exposure to chlorine, halogen compounds, silicones, sulfur or heavy metals can poison or damage the catalyst or render it nonfunctional. Dust in a flame arrestor or exposure to heavy oils and greases also can damage the sensor.
Electrochemical detection. In electrochemical detectors, molecules of the target gas react on the sensing electrode to generate a current. The devices are accurate and reliable, and respond rapidly to toxic gases like hydrogen sulfide (H2S), hydrogen chloride and carbon monoxide. The current generated by the cell correlates linearly to the amount of gas present in the atmosphere. Electrochemical cells consist of sensing, counter and reference electrodes within a single housing that also contains a small amount of electrolyte (the conductive solution). The reference electrode keeps the working electrode performing at optimum levels.
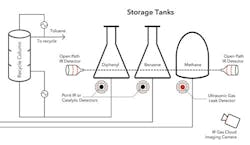
Figure 2. Using several gas-detection technologies is advisable for many applications such as hydrodealkylation of toluene.
These detectors offer fast response speeds and high accuracy, boast low power consumption, and can detect a broad range of toxic gases. However, they’re also highly sensitive to temperature and pressure, which are factors that affect the chemical reaction. The reaction speed, a primary advantage of this type of detector, decreases as temperatures decline. Additionally, electrochemical cells can handle a more-limited temperature range than other detectors. In addition, the cells have a pressure range of 10% of atmospheric pressure (i.e., 1.01 ± 0.1 bar); any pressure level outside of this range negatively impacts the gas measurement accuracy. They’re also not failsafe and perform poorly in prolonged exposure to dry environments (< 15% RH) or at low ambient temperatures (<-40°C).
Solid-state sensing. Solid-state sensors contain one or more metal oxides that are deposited as thin films onto an oxide substrate. A heater circuit increases the temperature of the film to an optimal point that produces the highest sensitivity and fastest response time to the gas to be detected. Biased electrodes embedded in the metal oxide evaluate the change in resistance, which is rendered into a gas concentration. Solid-state sensors must be exposed to H2S periodically. If not, their response rate goes down to several minutes, which is problematic.
While it originally was thought that solid-state sensors had many advantages, including their sensitivity to a wide range of gases and long life (as much as 10 years), it since has been shown that the technology is inferior for many uses. Solid-state sensors are prone to desensitizing over time if not exposed to the gas of interest. In some sites with high humidity, solid-state sensors may become unresponsive in as little as three months. Other disadvantages include high cross-sensitivity and high power consumption; these may not be evident in the short term, but will afflict the devices over time.
IR detection. IR detectors use two optical beams — an active beam having a gas-absorbing wavelength and a reference beam with a wavelength that isn’t absorbed by the gas — that follow the same optical path. Both wavelengths largely are impervious to such common environmental factors as carbon dioxide, nitrogen, water vapor and oxygen. IR gas devices can be point or open-path detectors for hydrocarbon gas. In open-path IR devices, the sampling path can extend to more than 100 m, in contrast to the <10 cm of point IR detectors.
All IR detectors are inherently immune to poisons and interferants and offer high sensitivity, low maintenance requirements, failsafe operation and easy installation. They also perform well in environments without oxygen or with enriched oxygen and can function in the continuous presence of gas. However, these detectors only can detect gases strongly absorbent within the IR spectrum. Plus, to detect gas, they must have large sample volumes; so if a gas leak doesn’t accumulate in the sample chamber, an IR device might fail to detect and respond to the leak. They also operate best within a narrow range of ambient conditions; exposure to dust, high temperatures and condensation can increase maintenance requirements and expenses.
Ultrasonic detection. The operating principle of ultrasonic gas leak detectors is that jetting gas from a high-pressure vessel or other pressurized system generates ultrasound (i.e., sound frequencies higher than those audible to humans) that, when detected by an acoustic sensor, provides a measure of leak rate. The speed of propagation depends on density and pressure. As a result, the velocity of sound varies with the medium. This is important to the detection of leaks.
Ultrasonic gas leak detectors can detect a variety of combustible, toxic or inert gases. In addition, they have short response times, governed by the speed of airborne sound, and aren’t influenced by the dilution of the gas cloud, which makes them ideal for use in windy areas. They also have no consumable parts and minimal maintenance requirements, and demonstrate robust failsafe operation.
However, they can’t determine the concentration of gas and are restricted to use with pressurized systems. Pressure leaks below 10 bar or 145 psi don’t produce acoustic emissions at levels substantially higher than the background noise in industrial environments (~50–80 dB). Additionally, ultrasonic gas leak monitors only can detect leaks produced by turbulent flow; at a minimum, the pressure behind the leak must be at least twice that on the other side for turbulent flow to occur. Acoustic sensing technology won’t detect vapors created from leaking liquids even though the vapors might reach flammable or toxic levels. Ultrasonic noise interference also can affect ultrasonic detectors. In a plant, such interferences can arise from electrical generators, pneumatic actuators, fans and other man-made or natural sources. Preventing false signals requires increasing the detection threshold, which may decrease sensitivity or effectively decrease response time.
IR gas cloud imaging. Originally developed for military applications, this is a technology to watch — but currently is far too expensive for general use. It uses an absorption gas imaging methodology. An IR camera images an area illuminated by IR radiation either from a laser or from the sun and other natural sources. In the video image, any gas that is present in the area shows up as a collection of dark pixels; the dark cloud formed by the agglomeration of pixels enables the rapid identification of gas leaks. It’s an excellent technology for wide field of view and long detection range. For example, some IR gas imagers can supervise entire sectors of a plant with detailed spatial resolution and allow for the characterization of hazards in ways unavailable to other detection techniques. With IR cameras, one can visualize the evolution of a gas cloud over time and determine whether the gas emanates from one or several sources and the direction of dispersal. At the same time, imaginers aren’t suited for small fugitive leaks and air current and humidity can afflict resolution.
The Need For Integration
As the brief rundown on the technologies makes clear, no single option can provide the sensitivity and response time required for every gas, nor meet the demands of every topology and monitoring distance. That’s why an integrated approach is optimal.
Catalytic (or electrocatalytic) detectors and fixed-point IR detectors are single point devices. They operate best at a monitoring radius of 0.5 to 2 m; so they should be placed near potential sources of gas leaks. By contrast, ultrasonic gas leak detectors protect larger areas. The devices have a radial coverage of approximately 452 m2 in a 12-m radius.
Figure 2 shows part of a flowscheme for the hydrodealkylation of toluene to produce benzene and illustrates how detection technologies can be integrated at a chemical plant. A recycle column separates toluene from unwanted diphenyl, while benzene and methane — separated earlier — are stored in tanks. Fixed-point or catalytic detectors are placed near pumps, valves and flanges. Ultrasonic detectors also are installed near the methane storage tank because methane is lighter than air and may not accumulate. The ultrasonic devices act as an extra line of defense should small leaks erupt into large fluxes of gas. Overlooking the entire area, an IR imaging camera provides protection against the release of large methane clouds.
Achieve Effective Monitoring
Several technology options are available today for gas detection at chemical and petrochemical plants. They all have their own benefits and drawbacks. The most effective approach typically involves integrating a few options together in different parts of the plant to meet the specific needs of the particular applications. You must consider several factors, including the types of gases needing monitoring, layout of the plant area, types and sizes of equipment used, whether the space is open or closed off, environmental conditions and many more. Once these requirements are understood, plant operators can build out a plantwide gas detection system that will help ensure critical plant safety.
EDWARD NARANJO is director of product management, Rosemount flame and gas detection, for Emerson Automation Solutions, Shakopee, Minn. Email him at [email protected].