Drying is a fertile area for problems, especially when working with organic chemicals. It’s a process that requires a delicate balance between heat input and mixing of the solids.
The first thing I consider when troubleshooting a solids process is the type of chemical or chemicals and unit operation involved. That’s because each one is unique. For example, when I hear a problem involving organics, I think “phase change.” Likewise, a problem with a screener shouts “particle size” or “particle shape” issues. Why do these come to mind? Every device has fundamental operating parameters. When you go outside these boundaries, bad things happen. For a drying operation, there is a delicate balance between keeping the particulate solids suspended and the generation of fine particles. For example, fluid beds do a fine job of suspending the solids but can overheat the particles, especially if they are organic chemicals. Often we would select a freeze dryer to limit the excess heat. However, pulling the solvent out too quickly can fluidize the solids or, worse, shear the solids in the capillaries.
While heat may be the enemy of organics in a fluid bed, the shear in a freeze dryer may degrade the organic to the same extent. Shear imparts excess energy into the particles to a similar extent as thermal energy. We had a sticky material and a low melting point. Freeze-drying sounded like a perfect fit. However, in trials, the particles melted, apparently due to the shear caused by the vapor flow. A flash dryer followed by a fluid bed that used low-humidity gas solved the problem.
Remember, it’s not temperature that drives drying but vapor pressure difference. So, when you plot your drying curve, always include tests that cover a wide range of humidity.
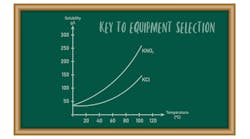
Crystals offer a whole new landscape for solids processing as they influence so many parameters downstream, such as dustiness and dissolution rate. At the heart of control is a good understanding of solubility and the mechanisms involved. The chemical you are trying to crystallize will dissolve given the right conditions, which can be a blessing when trying to minimize fine particles in the final product or a curse when trying to reduce dustiness.
As I mentioned in my previous column (“Vital Data for Solids Scale-Up”), solubility curves are required to troubleshoot crystallizers. It’s insufficient to have one curve but one for each of the polymorphs or solvates involved in the process. It’s not unusual to see the curves cross, as in the case I cited. In addition, some chemicals decrease in solubility as the temperature increases. The curve can even be flat.
One of the complications with solids, in general, is particle size because the physical properties change with a mixture of particle sizes. Fine particles dissolve faster, dry faster, filter slower and interact more with other particles than large particles (think hindered settling). One of the important properties to consider when diagnosing operating problems is the particle size distribution.
Many years ago, the best you could do to determine size distribution was a screen analysis. Unfortunately, particle behavior on a screen is not what happens in a fluid bed, crystallizer or pneumatic conveyor.
For example, plant managers once called me to help them after a customer rejected their product due to too many fine particles. The plant used screens to check the particle size, and it met the specifications. However, the customer used pneumatic conveying to unload the product, which generated excess fines, but we still had more fines in the product after transport.
Under a microscope, we observed the fines clinging to the larger particles at our plant. They were held together by a static charge that dissipated during transport to the customer releasing fine particles. Adding ionized air to the loading operation helped release the fines to the local dust control system, solving the problem. Also, the plant replaced its screen sizing with a laser-based system.
These short examples highlight unexpected characteristics of solids that can dramatically alter process outcomes, but maybe for solids, should be expected.