The catalyst can be reused multiple times without being deactivated.
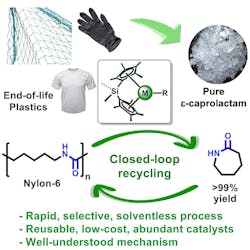
Researchers at Northwestern University, Evanston, Illinois, have patented an organometallic catalytic-based process that depolymerizes Nylon-6 under mild, benign solvent-free conditions with what they describe as unprecedented rates and selectivity.
Based at Northwestern’s School of Engineering and its Institute for Sustainability and Engineering, the researchers used a previously developed in-house catalyst that harnesses yttrium and lanthanide ions. When this was added to melted Nylon-6 samples the plastic fell apart, reverting to its original ε-caprolactam building blocks and leaving behind no byproducts.
“This solventless process operates with catalyst loadings as low as 0.04 mol % at temperatures as low as 220°C — the mildest Nylon-6 depolymerization conditions reported to date,” write the researchers in a recent issue of Chem.
The catalyst can be reused multiple times without being deactivated.
In addition to its high yield of monomers, the catalyst is highly selective and acts only on Nylon-6 polymers. As such, it could be used by industry to target Nylon-6 in large volumes of unsorted waste.
"If you don't have a catalyst that's selective, then how do you separate the nylon from the rest of waste?" asked Northwestern chemistry and engineering professor Tobin Marks, who is also the study's senior author. "You would need to hire humans to sort through all the waste to remove the nylon. That's enormously expensive and inefficient. But if the catalyst only degrades the nylon and leaves everything else behind, that's incredibly efficient," he added.
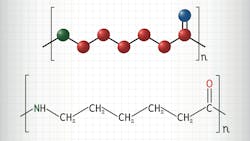
The researchers also investigated if they could create a fully circular end-of-life Nylon-6 economy by re-polymerizing recovered ε-caprolactam from a depolymerized waste fishing net. Using an anionic polymerization procedure, they obtained recycled Nylon-6 with a 99.3% yield.
Analysis revealed this Nylon-6 to be of higher quality than that of the original fishing net — prompting the researchers to point out that their new approach could both play an important part in environmental remediation and perform the first step in upcycling Nylon-6 wastes into higher-value products (Figure 1).
The Northwestern team says they have interest from potential industrial partners but did not respond to questions about the companies involved. Similarly, there was no response to inquiries as to whether a similar catalytic approach could be used to treat polymer types other than Nylon-6.
For now, the researchers are working with the Querrey InQbation Lab, Northwestern's technology accelerator facility charged with commercializing promising scientific and engineering discoveries.
About the Author
Seán Ottewell
Editor-at-Large
Seán Crevan Ottewell is Chemical Processing's Editor-at-Large. Seán earned his bachelor's of science degree in biochemistry at the University of Warwick and his master's in radiation biochemistry at the University of London. He served as Science Officer with the UK Department of Environment’s Chernobyl Monitoring Unit’s Food Science Radiation Unit, London. His editorial background includes assistant editor, news editor and then editor of The Chemical Engineer, the Institution of Chemical Engineers’ twice monthly technical journal. Prior to joining Chemical Processing in 2012 he was editor of European Chemical Engineer, European Process Engineer, International Power Engineer, and European Laboratory Scientist, with Setform Limited, London.
He is based in East Mayo, Republic of Ireland, where he and his wife Suzi (a maths, biology and chemistry teacher) host guests from all over the world at their holiday cottage in East Mayo.