The quest by leading pharmaceutical companies to achieve continued process verification (CPV) — or continuous process verification as it’s called in the European Union — has begun in earnest. Originally a guidance issued by the U.S. Food and Drug Administration (FDA) in 2011 [1], and subsequently by the European Medicines Agency (EMA) in 2014, CPV is intended to demonstrate that every step in a validated manufacturing process remains in the validated state.
This guidance not only impacts pharmaceutical manufacturers themselves but also likely will ripple through their supply chain, including chemical and specialty chemical companies.
Stage 3 of the guidance specifies statistical process control (SPC) and process capability analysis (PCA) as its foundation. This means that CPV goes beyond simply monitoring production processes to actually improving them. The ultimate goal is active process understanding and, if needed, proactive intervention and control. It is intended to provide information about process health — ideally in as close to real time as possible/practical — so a manufacturer can make changes to keep a process in the “sweet spot” and avoid producing off-specification and, therefore, potentially ineffective or, worse, dangerous poor-quality product. This includes extending that control to the inbound active ingredients and excipients from suppliers.
[callToAction ]
As a result, process engineers at chemical and specialty chemical manufacturers supplying pharmaceutical companies will need to satisfy increasing customer (and regulatory) demands to deliver “product to control” not just to specification. This will offer them opportunities to drive cost reductions and yield increases in their own processes using the same real-time analytics, visibility and control approach employed by their customers.
The good news is that many top chemical and pharmaceutical manufacturers — and their suppliers — for years have used an older sibling of CPV called enterprise manufacturing intelligence (EMI). These companies, by actively promoting their capabilities, stand to improve their position in the market.
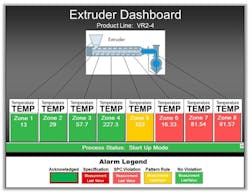
Figure 1. Staff at plant require data on particular unit to refresh fairly frequently, perhaps as often as once every half hour.
Given the prominence and critical nature of CPV along the entire supply chain, it’s important for suppliers — especially the process engineers directly responsible for manufacturing at those companies — to understand the opportunities and potential impact of the regulatory guidance.
Two Imperatives
Ultimately, two compelling forces are driving manufacturers to implement CPV. The latest research from LNS Research and MESA International [2] confirms that regulatory requirements for quality management are by far the biggest concern.
A recent article [3] notes: “Regulatory agencies will come to expect robust CPV programs rooted in sound process understanding… Inspection frequency may be influenced by the quality of a company’s CPV system and reported metrics for process health.”
The FDA’s guidance stresses the need to use data and acquired knowledge to continually improve processes by changing them based on the analytics-based root-cause analysis of problems. CPV, in turn, plugs directly into the quality-by-design framework as an ideal means to systematically identify and mitigate risks associated with product manufacture by continually monitoring, verifying and enabling immediate action for optimal process performance. CPV serves to provide ongoing verification of the process design and aids in enhancing process understanding [4].
The other key driver compelling manufacturers to implement CPV is competitive economic pressure. A recent article [5] predicts that companies — both brand manufacturers and contract service providers — that can adopt new technologies into their operations that result in better understanding, improved control and lower cost will come out winners during the turbulent times ahead.
The same process changes made to meet the regulatory guidance also enable manufacturers to identify and proactively address sources of process variation. That translates directly into ongoing process improvement, greater yields and more reliable delivery to the downstream supply chain. In turn, this leads to lower costs and higher margins, which is particularly important to companies with slim margins such as active materials and excipient suppliers, contract manufacturers and makers of generics and products that have lost patent protection.
These driving forces not only make delivering on CPV a strategic imperative for manufacturers but also increase the pressures to select technology that provides results quickly and reliably. To do so requires leveraging a manufacturer’s existing technology infrastructure while also enabling fast implementation (weeks not months or years), quick assimilation and understanding at all levels of the organization (operators to executives), easy scale-up to cover the enterprise, and delivery of quantifiable results immediately.
Fortunately, the FDA leaves the details of how CPV gets implemented to each individual manufacturer, as long as it’s done within the parameters of the original guidance. This allows companies the latitude to explore how leading manufacturers have achieved the equivalent of CPV.
Figure 2. Dashboard allows corporate staff to compare the performance of different plants making the same product.
As already noted, some of the top chemical and specialty chemical companies for years have used EMI. Its requirements [6] mirror those of CPV outlined by the FDA — namely, the ability to:
• aggregate/access data from many sources, including from other production sites;
• provide structure for the data that helps users find what they need;
• analyze these data;
• visualize the analyzed data to call attention to the most important information of the moment; and
• automatically transfer the analyzed data to the proper decision-maker to enable action.
These requirements deliver the key elements of CPV — improving process visibility, monitoring processes in real time, and applying analytics through the manufacturing process.
The Way Forward
With EMI providing the blueprint to achieve CPV, the challenge pivots to finding the smartest way to do it. Here, manufacturers can benefit from the experiences of those who have successfully deployed EMI.
Most of the functional aspects of CPV currently are met by more mature applications with established best practices including:
• flexibility — easy connection to multiple data sources, and delivery of role-specific dashboards from the same underlying data;
• timeliness — deployment in a matter of weeks instead of years; and
• scalability — from line or unit levels to enterprise level to cover multiple plants.
Now, let’s look at some points that deserve particular attention.
Data aggregation through direct connectivity. A company’s current validated systems, such as those for laboratory information management, enterprise resource planning, manufacturing execution, quality, historical data, etc., should not complicate the ability to deliver CPV.
The CPV system is an integral part of overall risk management and receives inputs from several other systems that support process design, development, qualification, nonconformance investigations, complaints, change controls, process data monitoring and raw material testing [3].
The usual approach for aggregating data across disparate systems has been to put all the data into a warehouse or data lake. However, that approach imposes the need to revalidate the system as well as the duplicated data (a significant issue by itself). That comes at a steep price in terms of time, personnel resources and financial commitment.
Instead, it’s better to leave the data where they were originally captured — in multiple existing validated data stores — and connect to them through industry-standard methodologies (e.g., OPC, ODBC, OleDB) or well-established application program interfaces to access the data directly for use by a global analytics layer. This leaves the data fully intact (and validated) with the original systems-of-record.
Just as critical is a system that lets you deal with all the different types of data (e.g., transactional, historized, sampled) — stored in multiple database technologies — required by CPV; these data likely will come from process development, clinical, process qualification and other full-scale production lots [3].
Data aggregation through direct data-source connectivity greatly simplifies the first critical step on the path to CPV/EMI, increasing the pace of implementation, deployment and value.
Data analysis. Manufacturing analytics and intelligence rank as the No. 2 and 3 software implementation priorities, according to a recent industry study. The top application of analytics is continuing manufacturing process improvement — a key attribute of CPV.
Analysis of data and reporting for CPV also may involve examination of existing process measurements and improved methods for data tracking and analysis beyond what typically is done for process validation [4].
When it comes to choosing which data to track and analyze, pharmaceutical manufacturers are ahead of their counterparts in other batch and process industries. They already have figured out what’s important for keeping processes on track and have been doing it for years — long relying upon critical quality attributes, critical process parameters and key performance indicators.
As already noted, the FDA’s guidance on analytics refers specifically to SPC and PCA, identifying them as the most appropriate for the widest range of data and users. SPC analytics engenders user confidence because it provides a low rate of false-positives, clarity of presentation, the ability to take action related to the signals and, thus, overall usefulness in increasing process understanding while decreasing operation risk.
The analytics techniques spelled out in the guidance primarily analyze variation, both short term and over time. Such analyses require data that are sampled properly for the statistical techniques, have adequate precision and can be readily queried from data sources that may include inappropriate data (such as historian data collected during startup, shutdown and process upsets).
Fortunately, SPC and PCA techniques are among the most robust and adaptable methods available. Recommending relatively proven and well-understood approaches avoids the difficulties and time typically required to implement esoteric statistical techniques and the creation and maintenance of complex models.
Dashboards. CPV involves not just monitoring and alarming processes with analytics but also visualizing and communicating what’s important at any given time to enable immediate intervention and action to maintain a steady production state. The key aspects of this are understanding what real time means for different roles and providing appropriate context.
• Real time. The results of an ongoing monitoring effort may be most impactful if they are reported to interested parties as close to real time as possible [3]. Experience with EMI shows that “real time” takes on different meanings depending on the role of the person interacting with a dashboard and how quickly that person needs to see a warning signal and respond to correct alarm-generating conditions. Sampling rates for process data are measured in minutes, not seconds, while update rates necessary for quality control stations and laboratory tests are much lower. This simplifies data integration and reduces hardware and network performance requirements.
For a process engineer, real-time dashboards refresh anywhere from every 30 minutes to once per shift, at the start of the day or on-demand for meetings and to assist problem resolution teams (Figure 1). This tactical view and alarming enables course corrections, optimizing across multiple variables and phenomena.
Corporate’s real-time view is usually strategic with daily or even weekly refreshes sufficing for product-related purposes and for comparing the economics of global production factors like product quality and yield across multiple plants making same product (Figure 2). However, alarm conditions for selected parameters critical to process health or safety will trigger notifications for immediate awareness and action as required.
• Context. Delivering analyzed data to the right people at the right time is only part of the equation. It is also essential to include as much context as possible with the data and to strive to increase the richness of the available information as the systems evolve to effect the right process and operational changes.
A manufacturer should establish a business process that addresses how often the results of the CPV program are reported, in what way the reporting is accomplished, and who the target audience is. The company should regard the CPV plan as a living document, updating it accordingly as process changes occur. Finally, a practice not specifically called out in the EMI definition — but a key and proven element of longer-term success and a best-practice across the process industries — is establishing an assignable cause/corrective action (ACCA) program; this corresponds to corrective and preventive action (CAPA), a mandated activity that most pharmaceutical companies have in place. It is essential that the CPV/EMI technology selected contribute to and integrate easily with existing CAPA activities.
Take The Right Steps
Because regulations do not dictate a particular way to deliver CPV, industry leaders face a challenge but also an opportunity to define how best to achieve it at their companies. EMI not only offers a clear established option to do so but also strengthens a manufacturer’s position in an increasingly competitive market.
Proven EMI implementation and execution blueprints provided by recognized leaders in the chemical industry greatly reduce the overall risk by codifying the technology requirements necessary for success that conveniently fit seamlessly in an existing validated environment.
PETER GUILFOYLE is vice president of Northwest Analytics, Portland, Ore. Email him at [email protected].
REFERENCES
1. “Process Validation: General Principles and Practices,” U.S. FDA et al., Washington, D.C. (Jan. 2011).
2. “Metrics That Matter,” LNS Research/MESA International, Cambridge, Mass. (Apr. 2016).
3. Boyer, M., Gampfer, J., Zamamiri, A., and Payne, R., “A Roadmap for the Implementation of Continued Process Verification,” PDA J. of Pharm. Sci. & Tech. (Jan. 2016).
4. Payne, R. and Fleming, J., “Continued Process Verification for Biopharma Manufacturing,” BioPharm Intl., Vol. 27, Iss. 10 (Oct. 2014).
5. Gaspar, F., Gil, M., and Matos, N., “Continuous Processing: Meeting the Need for New Manufacturing Strategies,” Amer. Pharm. Rev. (Jan. 2016).
6. “Enterprise Manufacturing Intelligence Helps Global Manufacturers Raise Performance and Responsiveness,” AMR Research, Boston (Nov. 2003).