Evaluate SMB Chromatography for Your Separation
Simulated moving bed (SMB) chromatography is a well-established separation and purification technique useful in commodity and specialty chemical manufacturing. The basic SMB process scheme was patented in 1961 by Broughton and Gerhold at UOP [1].
Over the years, companies have commercialized a number of diverse applications including isolation of p-xylene from mixtures of C8 aromatics, separation of fructose from glucose in the production of high-fructose corn syrup, and purification of various pharmaceuticals such as chiral compounds and biopharmaceuticals [2–6]. A previous article in Chemical Processing, "SMB Chromatography Offers Real Attractions," by Kathleen Mihlbachler and Oliver Dapremont [6], available online at www.ChemicalProcessing.com/articles/2005/538.html, discusses the technology's potential, particularly in the pharmaceutical industry.
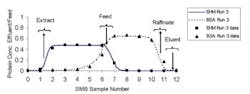
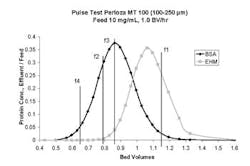
In this article, we'll review the basic SMB process scheme with a focus on flow rate requirements, and outline a way to determine whether SMB chromatography makes sense for your application, summarizing methodology we've found useful in our own work [7]. The approach we recommend involves analyzing single column chromatograms or pulse tests. It's an extension of a method introduced by deRosset, Neuzil and Korous in 1976 [8], and enables convenient evaluation of various media properties and operating conditions, such as media type, pore structure, temperature and choice of elution solvent.
As we'll explain, SMB flow rate requirements can be assessed by analysis of elution peaks. For more information about SMB technology in general, see the reviews of process fundamentals by Ruthven and Ching [2], Wankat [3], and Nicoud [4], and discussions of various factors involved in design and scale-up by Pynnonen [5] and Chin and Wang [9]. Although various modifications to the basic SMB processing scheme have been introduced over the years [9], the basic process remains popular.
THE BASICS
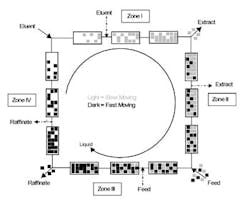
The classic SMB chromatography process separates a feed stream containing two or more dissolved solutes into two effluent streams — a stream rich in the relatively slow-eluting solute or solutes (called the extract) and a stream rich in the faster-eluting solutes (the raffinate). An SMB process approaches the efficiency of a true countercurrent solid/liquid process while avoiding problems associated with particle movement. Compared to standard pulse injection (batch) chromatography, SMB operation minimizes product dilution and maximizes productivity of separation media. It can't improve purity or recovery achievable by batch operation but offers much greater operating efficiencies that dramatically reduce capital and operating costs for commercial-scale implementation.