Many chemical plants across the globe use seawater as a cooling medium. Depending on specific plant cooling requirements, these systems can demand several thousand gallons of fresh seawater makeup per minute. The high volumes of makeup water used represent a significant expense on two fronts: the energy to pump the water and the chemicals to treat it. So, optimizing both seawater demand and chemical treatment strategy is critical.
However, plants that rely on seawater for cooling have struggled to do this. For years, conventional seawater cooling programs have used outdated chemistry guidelines that were adapted from freshwater cooling systems or oilfield brines. These adaptations can be imprecise and have led to an unending battle against a range of negative outcomes: high chemical costs, reduced heat transfer efficiency, unscheduled plant shutdowns, lost production, steep maintenance expenses for electrochlorinator units, and excessive water pumping.
Fortunately, new approaches that use digital monitoring and computerized saturation models to optimize cooling programs have emerged. For instance, SUEZ Water Technologies & Solutions’ new OptiSea chemical treatment program combined with its proprietary MonitAll digital monitoring device have enabled several advances on this front.
Here, we’ll look at common problems that arise from the use of faulty models in plants relying on seawater for cooling, and how new digital strategies are overcoming these difficulties.
Minimizing Seawater Makeup
Seawater cooling systems at chemical plants typically use a cooling tower to provide some level of water reuse. As a secondary benefit, they are designed to minimize thermal discharge that could damage coral reefs.
Devising an operating strategy for seawater cooling systems isn’t a simple task. Seawater chemistry varies significantly across the globe. This means different systems may require disparate types or dosages of treatment chemicals, and the maximum amount of water reuse will differ from plant to plant. A treatment strategy also must consider other plant-specific operating conditions, including operating temperatures, water flow velocities and environmental discharge limitations.
The calculation of makeup demand is an essential baseline inquiry that underpins many other decisions. The demand depends upon the amount of water reuse, which is measured by a dimensionless number called “cycles of concentration,” C.
C = makeup flowrate/blowdown flowrate (1)
It also can be expressed in terms of the salt concentration of the makeup and recirculating water (blowdown) streams. For practical purposes, conductivity measurements often are used:
C = blowdown conductivity/makeup conductivity (2)
To safely minimize makeup water demand and pumping costs, you must know the upper operating limit of C to avoid scale formation in heat exchangers. Then, you must operate the cooling tower system accordingly to ensure you are maintaining the tower water chemistry in a safe range.
If the plant is operating at constant heat load, as C increases, the flow rates of both makeup and blowdown decrease. Therefore, operating at higher C means reuse of more water. However, as seen in Equation 2, operating at higher C also increases the salt content of the water. If C is raised too high, some dissolved salts (primarily calcium salts) can precipitate out of solution to form scale on heat transfer surfaces throughout the plant, reducing heat transfer efficiency. For these reasons, it’s desirable to design the chemical treatment program and operating conditions such that C is as high as possible without risking scale buildup in the heat exchangers.
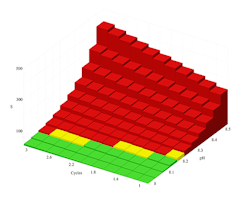
Figure 1. Calcium carbonate solubility curve at 120°F points out risk of precipitation when pH is 8.2 or greater even at low cycles of concentration.
In the past, due to treatment chemical limitations, seawater cooling treatment programs only could operate at a C between about 1.2 and 1.7. Recent advances now allow some systems to operate at a C of 3.1. This reduces makeup water demand by nearly 50% and cuts pumping costs proportionally as well.
Properly Modeling Saturation
Once operating parameters for the cooling tower are established, attention can turn to chemical treatment programs. Until recently, such programs for seawater cooling systems relied heavily on the use of saturation “indices” to predict scaling tendencies for calcium carbonate (CaCO3) and calcium sulfate (CaSO4), the two most common deposits found in seawater-cooled heat exchangers. These models attempted to predict chemical treatment rates and safe operating concentration ratios for seawater cooling systems using open recirculating cooling towers.
Unfortunately, traditional equilibrium models don’t work effectively in seawater cooling systems due to the high ionic strength of these waters. Using an inaccurate model to develop a treatment strategy leads to predicted chemical treatment or cooling tower blowdown rates that are either too high or too low. This could result in high water usage, excessive treatment cost or a loss of heat transfer efficiency in the plant.
Calcium carbonate is by far the most common scale encountered in seawater cooling systems. To define safe operating conditions, some water treatment consultants still employ commonly used saturation indices such as the Langelier Saturation Index (LSI) or the Stiff-Davis Stability Index (SDSI) to predict calcium carbonate solubility. However, these indices aren’t designed for seawater and don’t consider the complex interaction of ions. The LSI was developed for fresh potable water systems and the SDSI was designed to predict calcium carbonate scaling potential in oilfield brines. They don’t perform well in seawater and, so, neither should serve as a basis for designing a chemical treatment strategy for seawater cooling systems.
That has led some consultants to either develop their own equilibrium modeling tools or use commercially available software to predict calcium carbonate solubility. These models rely on an activity coefficient, a factor used to account for deviations from ideal behavior in mixtures of chemical substances. For aqueous solutions, several available equations predict activity coefficients as a function of ionic strength.
Unfortunately, published activity coefficient curves were developed for ionic strength solutions typically <0.1 and, at most, <0.5 (molal). Cycled-up seawater has an ionic strength >1. These activity coefficient curves don’t work well for seawater and can provide misleading results if used to design a treatment program for a seawater cooling system. Also, these models don’t accurately predict the pH and alkalinity relationship in seawater or “cycled” concentrated seawater. Knowing this relationship is crucial to performing accurate calcium carbonate solubility calculations.
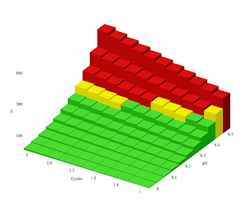
Figure 2. A proprietary treatment for 120°F seawater dramatically expands safe operating conditions to allow operation at higher cycles of concentration and pH.
To overcome the problems with published equilibrium models, a proprietary software package was developed. This software incorporates accurate activity coefficient and pH calculations. It also accounts for the effect that anti-scalants and treatment chemicals exert on calcium carbonate formation and utilizes empirical data to support the model.
Figures 1 and 2 illustrate the output of the proprietary saturation modeling software. Figure 1 shows the solubility of calcium carbonate at 120°F in Caribbean seawater with no chemical treatment applied. The data indicate that without treatment, calcium carbonate will form. The red bars point out that calcium carbonate will be a problem even without cycling if the pH is over about 8.1. Because natural seawater has a pH of roughly 8.1–8.4, using untreated seawater in cooling systems wouldn’t be advised.
Figure 2 shows that a proprietary anti-scalant product allows safe operation of this system at a C of up to 3 if the pH remains below 8.4.
These graphs not only visually represent the safe operating window for these particular chemical treatment programs — but also shed light on the remarkable room for improvement at many seawater cooling facilities.
Adopting Best Practices
The primary function of chemical treatments used in seawater cooling systems is to inhibit mineral scale formation on heat transfer surfaces. Some treatment programs also incorporate chemicals designed to disperse suspended solids, which minimizes potential for heat exchangers to become fouled with inorganic debris (i.e., mud, sand and silt). Mineral scales and foulants provide an insulating layer on heat exchanger surfaces and reduce the heat transfer efficiency. The goal of the chemical treatment program is to maintain clean heat exchangers, free of scale and foulants, so that the process is more energy efficient. Unlike in freshwater cooling systems, corrosion typically isn’t a problem in seawater cooling systems because these systems employ corrosion-resistant metallurgies and often utilize lined transfer piping in the water distribution circuits. For these reasons, seawater cooling systems usually don’t require corrosion inhibitors.
In the past, seawater cooling treatment programs primarily focused on scale-control treatment chemicals, often using phosphorous-based anti-scalants. These phosphorous-based treatments can effectively control scale but they are coming under increased environmental scrutiny due to their potential to cause algal blooms and damage coral reefs. While governmental restrictions on phosphorous discharge aren’t yet in place globally, end users should seriously consider the public relations issues that could result from an unsightly algal bloom created by unnecessary discharge of phosphorous.
Phosphorous-based anti-scalants have had a long history of success in seawater cooling applications. For this reason, they remain in use at many plants. However, new products that aren’t based on phosphorous chemistry allow chemical plants to operate at a higher C than possible with phosphorous-based treatment chemicals. Doing so can provide substantial water savings and avoid the potential for algal blooms in plant outfall.
Many available treatment formulations include a fluorescent dye that enables the user to check the product concentration in the cooling water. Fluorometers designed to detect these dyes are available commercially and test procedures are easy to perform. However, the inclusion of dye components adds significant cost to the program and provides questionable value. You never can recover 100% of the dye in seawater samples; the recovery rate typically varies from 69% to 80%. This means that actual treatment dosages could be as much as 31% higher than necessary. Without the dye and without the ability to analyze the product concentration in the cooling water, you would never really know if you dosed enough chemical to avoid a failure. Given that some systems use several hundred thousand dollars in treatment chemicals annually, a 31% overfeed represents a substantial unnecessary expense. Moreover, the potential costs associated with a failure could be substantially higher, up to 10 times or more. Neither of these situations, to overfeed or underfeed, are acceptable.
Instead of feeding the chemical and testing for an ingredient, plants should adopt “smart” chemical feed pumps — now available at a nominal cost — to ensure the system always gets the correct amount of treatment chemical. These pumps continuously monitor actual chemical application rates and can automatically adjust rates based on system flowrates. Real-time data from these pumps can go to a distributed control system or cloud-based data management system to provide early warning alarms in the event of an immediate pump failure or, the more challenging to detect, slow failure over time. Pump repairs, of course, then must occur expeditiously, so chemical treatment can resume as quickly as possible.
Retrofitting existing chemical feed systems with smart pump technology is easy; it simply involves choosing a pump manufacturer, pump size (maximum flowrate and desired turndown capability), selecting the correct materials of construction based on the chemical product to be added to the cooling system, and ultimately replacing existing pumps with the new pumps. Making effective use of the technology and its added benefits may require installing an upgraded chemical feed controller and a connection to a cloud-based data management system, such as SUEZ’s InSight, to gain visibility to critical information reported by the new smart pump technologies. Water treatment companies typically offer these systems (pumps, controllers, data management, etc.) as a complete package to ensure successful implementation of the technology.
Monitoring Performance
Cooling water heat exchangers at chemical plants rarely are outfitted with enough flow and temperature instrumentation to enable real-time monitoring of heat transfer performance. To make up for this deficiency, some plants perform periodic temperature and flow checks or utilize process simulation software to model heat exchanger performance and cleanliness. However, these periodic checks don’t always identify problems in time to avoid significant losses in heat transfer and efficiency.
Advances in digital monitoring can help optimize the chemical treatment strategy. A digital probe can deliver data to the cloud on a constant basis and can alert personnel by sending text messages or emails if operations fall outside established parameters. While multiple heat exchanger simulators exist, the only technologies that provide enough information for a chemical operation are those that give a real-time analysis of cleanliness factor. Typically, these digital systems monitor heat transfer across a known surface and provide a real-time calculation of a cleanliness factor for the probe, reported in a cloud-based data management system. Real-time or near-real-time data offer early warning and enable proactive adjustments to the system or chemical treatment program to prevent costly deposition in heat exchangers.
Moving Forward
Until now, chemical treatment strategies for seawater cooling systems suffered from several deficiencies. While obviously there’s no “one size fits all” treatment approach for seawater cooling systems, a comprehensive, cost-effective and technically sound treatment strategy should:
1. Use the most cost-effective and environmentally acceptable chemical treatment.
2. Precisely control the chemical dosage.
3. Minimize the amount of seawater usage with respect to chemistry and environmental discharge limitations.
4. Measure cooling system heat-transfer performance to confirm results and provide an early warning if problems occur.
Faulty assumptions lead to imperfect treatment strategies. By using sound saturation models that function accurately over a wide range of seawater cooling applications, plant operators can get accurate, optimized programs that are custom-tailored to their specific plant operating conditions.
ROBIN WRIGHT is a technical consultant for SUEZ Water Technologies & Solutions in Ponte Vedra, Fla. Email him at [email protected].