Extracting Critical Materials From Gas and Oilfield Wastewaters
Oil and gas production in the U.S. generates tens of billions of barrels of waste fluids annually, according to the U.S. Environmental Protection Agency.
Historically, these produced waters have been managed primarily through injection in Class II wells, but in several states, injection wells have been associated with earthquakes. This challenge – combined with water-resource scarcity in certain geographic regions – has resulted in an increasing demand for above-ground wastewater treatment and reuse.
The produced waters, depending on their geographic location, often contain appreciable levels of critical materials, such as lithium. Effective extraction of these elements during wastewater treatment can generate additional profits (e.g., a concept called wastewater valorization) and support U.S. domestic supply chains and manufacturing.
A group of researchers at the U.S. Department of Energy’s Argonne National Laboratory (Argonne) are developing a patent-pending separation process to enable more effective extraction of lithium from gas and oil field wastewaters.
Existing lithium-extraction processes developed mainly for lithium mining from geothermal brines or salt lakes, involve time-consuming solar evaporation and costly precipitation and filtration steps. Direct lithium extraction (DLE), which selectively captures lithium out of a multi-ingredient liquid stream, can be more energy efficient and environmentally sustainable.
Early work in DLE has primarily relied on adsorption technologies, in which selective sorbents – such as ion-imprinted polymers, molecular sieve ion-exchange resins and intercalates (e.g., MnOx, TiOx, AlOH) – are used. Another method involves liquid-liquid extraction, which relies on specific solvents containing lithium binding organic materials like crown ethers, cyclic siloxane and ionic liquids. These methods, although effective, often require element desorption from adsorbers (called regeneration) by organic solvent wash, causing extra cost. By comparison, membrane separation, a common method used by the water industry, is more energy efficient and environmentally sustainable. However, commercial membranes are usually ineffective in separating cations of similar sizes and charges.
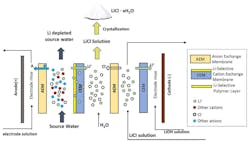
Argonne’s patent-pending separation process combines concepts from sorbent and membrane technologies to enable a novel continuous and selective extraction of lithium from industrial wastewaters without frequent regeneration. The core component of this process is a hybrid cation exchange membrane (CEM) that contains a lithium-selective polymer layer and a lithium-transport CEM layer (Fig. 1).
When source wastewater is introduced, lithium is preferentially bound to the hybrid membrane, while the other unwanted cations are rejected. Upon applying an external electric field, the captured cations are transported across the membrane to the product compartment, producing a concentrated liquid stream of lithium ions and counter anions introduced to the process for charge neutralization (like chlorine ions), which is then crystalized to generate lithium salt products such as lithium chloride (LiCl). Depending on the application, this process can either generate LiCl as an end product for lithium metal production or further convert LiCl to lithium hydroxide (LiOH) for lithium battery cathode production through a second bipolar membrane electrodialysis (BPED) step.
This technology is source agnostic. In addition to gas and oilfield hydraulic cracking produced waters, the process can also be applied to geothermal brines and battery recycling processed waters. Additionally, the base monomer of the lithium-selective polymer layer can be modified to adapt to different cation sizes, making the technology a platform technology capable of extracting more than one element. The functional groups within molecular structure can also be modified to drastically “fine-tune” element selectivity to further tailor cation selectivity.
In addition to the base monomer unit being tunable, the polymer structure of the ethers can also be adjusted to optimize polymer processibility, lithium-ion transport and other membrane properties like water uptake, mechanical strength and chemical stability.
The Argonne team has observed complexation of lithium with a customized monomer unit and developed films out of this base material. The next step is to integrate the ether layer to a CEM to demonstrate a selective electrodialysis process. The team believes that once fully demonstrated, this new membrane extraction process will help the lithium mining companies, oil and gas production companies and wastewater treatment facilities generate extra profits.
As a federally funded research and development lab, Argonne is looking to team up with commercial partners interested in co-developing and deploying this emerging membrane technology to capture value of critical minerals such as lithium from industrial waste waters. The Argonne team can accelerate membrane technology development by leveraging some of the world’s most innovative manufacturing research facilities, such as the advanced X-ray and nanomaterial characterization techniques at Argonne’s Advanced Photon Source and Center for Nanoscale Materials. Additionally, Argonne’s Materials Engineering Research Facility has specialized capabilities in materials and membrane synthesis scale-up capabilities.
Argonne can also offer industry partners evaluation of a technology’s manufacturability and economic viability through the technoeconomic analysis IMPACT (Innovative Manufacturing and Processing Assessment Calculating Tool) model and life cycle assessment GREET (Greenhouse gases, Regulated Emissions, and Energy use in Technologies) model.
Argonne’s advanced materials capabilities and intellectual property are available to companies through research and development projects and technology licensing. Argonne invites additional collaboration with the private sector to tailor the lab’s direct membrane lithium extraction technology to address specific industry needs. To learn more, visit www.anl.gov/partners.
About the Author

Yuepeng Zhang
Dr. Yuepeng Zhang is a principal materials scientist at Argonne National Laboratory. She has a Ph.D. in materials science and engineering from McMaster University in Canada. Yuepeng’s current research focuses on nanomaterials and membrane technology development for water and gas separation, critical mineral recovery and battery and fuel cell electrode and separator applications. Residing in Argonne’s Materials Engineering and Research Facility, Yuepeng’s team also devotes effort to process innovation and scale-up based on additive manufacturing and roll-to-roll processing, such as roll-to-roll electrospinning, electrospray, slot-die coating and screen-printing. Before joining Argonne, Yuepeng worked as a principal engineer for Western Digital Corp. in San Jose, California.